Test smarter, not harder: Boosting quality and reducing avoidable costs through laboratory stewardship
To take the test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
1. Define laboratory diagnostic stewardship.
2. Describe outcomes of an organization’s stewardship program.
3. Identify actionable test utilization practices.
Hospitals across the United States are facing significant financial pressures, with operating margins experiencing fluctuations in recent years. Combined operating margins decreased from 8.9% in 2021 to 2.7% in 2022 before increasing to 5.2% in 2023. However, both combined operating margins and total margins have remained below 2019 pre-pandemic levels in 2023.1
There are several factors that have contributed to these financial difficulties, including:
- Lower reimbursement rates: Hospitals have experienced reduced payments from government payers (Medicare/Medicaid) and commercial insurers, impacting overall revenue streams.
- Rising labor expenses: Labor costs, which generally account for about half of a hospital’s total expenses, have risen significantly, driven in part by increased dependence on contract labor agencies.2
- Higher costs for medical supplies and diagnostic equipment: Hospitals have faced increased operational costs due to rising expenses related to prescription drugs and supplies.3
- Shifts in patient care from inpatient to outpatient settings: The transition to outpatient care has led to reduced inpatient volumes, affecting top line revenues for hospitals.
- Increased regulatory compliance costs: Hospitals must invest in compliance measures to meet regulatory requirements, adding to operational costs.
Considering these financial constraints, hospital leadership must find innovative ways to reduce operating expenses while sustaining and/or increasing the quality of care that is being offered. One opportunity for cost savings lies in optimizing laboratory test utilization through stewardship programs.
Diagnostic stewardship
Laboratory and pathology diagnostic stewardship refers to the coordinated effort to ensure that diagnostic tests are used appropriately and effectively to reduce unnecessary testing, reduce costs, and maintain or improve patient outcomes. Advancements in laboratory medicine have increased the availability of complex and specialized tests, and overuse of laboratory tests has been a growing problem in hospital settings, contributing to rising healthcare costs. Factors such as clinical routines, lack of cost transparency, and the convenience of electronic health record (EHR)-based ordering play significant roles in this overutilization.4 By integrating best practices and evidence-based guidelines, laboratory stewardship programs (LSPs) assist hospital systems in managing diagnostic utilization effectively amid tightening financial margins, rising operational costs, and regulatory compliance challenges.
Kaiser Permanente, Greater Southern Alameda Area Network
Kaiser Permanente is one of the largest integrated healthcare systems in the United States, serving over 12 million patients across eight states and a federal district. With over 35 hospitals and over 600 medical offices, it is known for its innovative care models, such as the early adoption of EHRs and value-based care initiatives. The organization is also committed to sustainability, implementing various environmental and healthcare stewardship programs to improve efficiency and patient outcomes.
The organization recognizes the importance of LSPs as a critical strategy for improving healthcare efficiency and reducing unnecessary diagnostic testing. To formalize these efforts, it established the Laboratory & Pathology (L&P) Utilization Committee, which serves as an advisory body dedicated to defining best practices in ordering laboratory diagnostics. The committee’s primary objectives include reviewing test utilization, establishing appropriateness criteria, minimizing variations in diagnostic testing, and reducing external testing costs through evidence-based decision-making.
Stemming from the LSP initiative, Kaiser launched the “Test Smarter, Not Harder” initiative (the Initiative), which focuses on improving laboratory efficiency within its Greater Southern Alameda Area (GSAA) network. The Initiative has three broad focuses: first, those tests currently being conducted externally that could feasibly be performed within the internal laboratory network; second, tests already being processed internally but which might be achievable at a significantly lower cost through alternative test choice; and third, tests —conducted internally or externally — that may be clinically unnecessary and could be safely avoided all together.
This article summarizes the first focus of the Initiative: leveraging a data-driven approach to identify tests being conducted externally that can be internalized. A key component of the project is to centralize laboratory utilization data that is currently fragmented across multiple laboratory units within the GSAA network and develop an organizational framework for conducting these evaluations regularly.
Initial approach
This project followed a structured, multi-step approach grounded in data analysis and stakeholder engagement. The initial step involved developing a thorough understanding of the modalities of laboratory test collection and processing across the GSAA network, which include four primary laboratory and pathology centers located in Sleepy Hollow, Union City, Fremont, and San Leandro. We worked with the regional laboratory operations team to collect and collate laboratory test utilization data across all four sites for the 2024 calendar year. Once the data sets were consolidated, we conducted a detailed review to understand how the data had been stratified and whether tests outsourced to external laboratories could be reliably identified.
We then analyzed the dataset to identify the top 10 tests that were most frequently sent outside the network. These tests would be selected as priority targets for potential internalization. With this data in hand, we would then collaborate with the finance team to assess the direct costs associated with outsourcing these tests, providing insights into the financial burden they place on the system.
With this foundational analysis in place, we conducted stakeholder interviews across the laboratory, operations, and finance departments to understand the workflow implications of internalizing these tests. This pro forma assessment aimed to estimate the potential cost savings associated with internalizing the selected tests. Finally, based on the findings from the data analysis and stakeholder consultations, the project would culminate in the development of a scalable recommendations that would enable the health system to replicate this LSP on an annual basis, ensuring ongoing optimization of diagnostic services across the GSAA network.
Laboratory utilization data
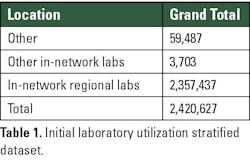
When we first looked at the laboratory utilization data from 2024, we observed that the GSAA network had made over four million test orders. Of these tests, we were able to extract that ~41.4% of these tests were classified as “send-in,” and 58.6% of them were “send-out” tests. Additional data allowed us to stratify the “send out” data into segments which reflected the location where the tests were processed: in-network regional labs, other in-network labs, and other. From this stratified data, the set that we were most concerned with were the tests classified as “Other,” as these were the tests that were sent to labs outside the health system network.
We determined that only ~2.46% of these tests that were classified as “send out” were actually tests that were sent to reference labs outside the network. Table 1 highlights the breakdown of the number of tests and their respective stratification.
With this data in hand, we wanted to get a broad picture of the financial implications of having just shy of 60,000 tests being sent out of the network. In our conversations with the finance team, we were able to determine that a total of $16.5 million was spent across the year on testing by the GSAA unit, of which ~$5.8 million were attributable to tests sent out of the network.
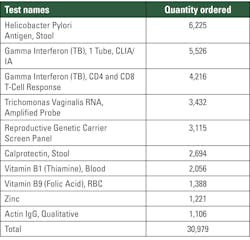
Our analysis unearthed the top ten tests that were being sent out of the network from the GSAA unit. These are listed in Table 2.
With this information, we see that these tests account for just shy of 50% of the tests being sent out of the network, and the data collected from the finance team reflected that total expenditure for conducting all these tests was $1.58 million.
Stakeholder interviews
The structured interview with clinicians, laboratory staff, and administrative stakeholders across the GSAA network revealed several recurring themes and nuanced insights regarding laboratory test ordering, data flow, and stewardship practices. The following synthesis highlights the most prominent findings:
- Limited physician awareness of test routing: A consistent theme was the lack of awareness among physicians regarding where laboratory tests are ultimately processed. Multiple clinicians emphasized that, aside from a few exceptions (notably “miscellaneous reference tests”), they generally do not know whether a test order is fulfilled internally, by a regional lab, an in-network lab, or to an external reference laboratory. Test routing is largely determined by pre-set options within the EHR system.
- Reliance on “smart set” test ordering: The interviews revealed a common trend of widespread reliance on standardized order sets or “smart sets” within the EHR system. Physicians across specialties routinely use these smart sets to streamline the ordering process for common clinical scenarios, such as well-child visits, prenatal care, or standard admissions. These order sets are typically pre-configured by clinical teams and are designed to reduce cognitive burden and improve order processing times by allowing providers to select a comprehensive panel of recommended tests with minimal manual input.
- Data fragmentation and manual processes: Stakeholders from both laboratory operations and information systems teams highlighted significant challenges in data extraction and consistency. The process of identifying the misclassification of the “miscellaneous reference labs” tests not being captured in a standardized way across the GSAA network reflects this (See Limitations section below). This has led to a reliance on tedious data cleaning and reconciliation, increasing the risk of errors in data analysis.
- Clinical department–specific ordering patterns and legacy practices:
Clinical departments exhibit distinct ordering behaviors:
● Pediatrics: Orders a high volume of tests, often to avoid repeat visits for children, which drives up external test utilization numbers for the department. The care model prioritizes minimizing patient inconvenience, sometimes at the expense of test stewardship.
● OB/GYN: Most tests ordered are already conducted internally, apart from state-mandated genetic screenings. For which, genetic counselors or maternal-fetal medicine specialists typically handle external orders, which fall under the “miscellaneous reference lab” test category. In this department there is a formal steering committee overseeing prenatal genetic test orders, and some tests are charged to the genetics team rather than to the L&P team budget.
● Gastroenterology: Relies on long-standing legacy ordering processes. Certain tests (e.g., Prometheus panels) are ordered out of habit, and there is little awareness or incentive to change these patterns. Over-ordering of specific tests like calprotectin or H. Pylori was noted, with suggestions that more critical evaluation of the clinical necessity of ordering these tests is warranted.
- Existing stewardship mechanisms: Some departments have implemented stewardship strategies, particularly within the OB/GYN and high-risk pregnancy care. These include:
● A combination in the use of smart sets along with a decision support tool in the EHR, designed to streamline ordering and reducing unnecessary tests.
● Hard stops and prompts for certain tests to ensure appropriateness.
● Oversight by genetics teams and steering committees for high-cost or high-volume genetics tests.
Overall, from these insights we can say that the workflow for laboratory test ordering is characterized by a high degree of automation, limited clinician visibility into test routing, and clinical departmental-specific adaptations. The interviews highlight both the efficiency and the opacity of the current system, suggesting that greater transparency and ongoing review of ordering protocols could further optimize test utilization and support laboratory stewardship goals.
Finally, the stakeholder interviews also provided valuable, practical recommendations on which laboratory tests could be prioritized for internalization within the GSAA network. Clinicians and administrative staff consistently identified several high-volume or frequently ordered tests that, if brought in-house, could yield significant cost savings and operational efficiencies.
Discussion & recommendations
The findings from this project reveal significant and actionable insights into laboratory tests utilization and stewardship within the GSAA network. The data and stakeholder interviews collectively highlight the dual challenge of optimizing test ordering practices while navigating substantial data and workflow barriers. These challenges are not merely operational inconveniences; they have direct implications for cost efficiency, clinical quality, and the ability to implement sustainable LSPs.
Based on the findings, several recommendations have emerged:
- Standardization and integration of data systems: We recommend that the GSAA network develop systemwide definitions and automated categorization for send-out tests, including “miscellaneous reference lab” tests. This will reduce the administrative burden for reconciliation of volumes, improve cost tracking, and enable more accurate stewardship analysis.
- Improve clinician cost transparency and decision support systems: Modify EHR interfaces to provide real-time feedback on test routing and costs at the point of order entry. Incorporate decision support systems or “hard stops” for high-cost or high-volume tests, as already piloted in the OB/GYN department, to encourage evidence-based ordering and reduce unnecessary externalization of tests.
- Prioritize internalization of high-volume ordered tests with predictable order patterns: Focus on internalizing those tests with predictable, high demand - such as cystic fibrosis carrier screening, H. Pylori, and calprotectin. This approach should be supported by clinical consensus.
- Establish a GSAA multidisciplinary stewardship committee: Create a standing committee with annually rotating representation from clinical, laboratory, information technology, and finance departments to oversee test utilization patterns and monitor impact of stewardship interventions. Regular review (e.g., annual) and feedback cycles are critical for sustaining improvements.
- Establish clinical department level stewardship committees: To complement the multidisciplinary stewardship committee, establish a structured process for annual review of EHR smart sets by departmental stewardship committees. This approach builds on existing efforts while addressing gaps identified in specialties. In addition, departmental stewardship committees will provide sub-departmental context to test ordering patterns to ensure there is a weighted criteria-based recommendation when required.
- Ongoing education and communication: Finally, the GSAA network should implement educational initiatives to inform clinicians about internal testing capabilities and impact of their ordering practices. Foster a culture of stewardship through regular updates and transparent reporting of utilization data.
Limitations
This project faced several limitations. First, the reliability of quantitative analysis was constrained by inconsistent data entry and hard coding, particularly for “miscellaneous reference lab” tests. We discovered that a portion of these tests were technically sent outside the health system network but were not captured in the official “send-out” section of the data. These tests, totaling approximately 2,298, were labeled as miscellaneous and stored in the “send-in” section of the data. The need for extensive data cleaning introduced a risk of error in data integrity. In addition, the lack of analysis of this data highlights the possible limitations in the recommendations offered.
Second, the interview sample, while diverse, may not be a complete representation of perspectives of all clinical departments. This means that there needs to be a more representative sample before the insights can be generalized.
Third, the analysis focused primarily on cost and volume outcomes, without direct measurement of clinical impacts such as turnaround times or patient outcomes. Finally, the findings are specific to the GSAA network and may not be fully generalizable to other regions or the entire health system with different organizational structures or EHR configurations.
Conclusion
In summary, this project demonstrated that meaningful LSPs require more than identifying high-cost tests; it demands investment in data infrastructure, workflow transparency, and interdisciplinary collaboration. These findings echo national trends where stewardship success is often linked to targeted, departmental-driven interventions and strong governance structures. Addressing the highlighted challenges will not only foster a culture of continuous quality improvement, but it could also result in financial savings and care quality excellence. By implementing the recommended strategies, the GSAA network can position itself as a leader in LSPs and set a replicable standard for the rest of the health system and other integrated healthcare systems in the country.
We would like to acknowledge and thank the laboratory staff and leadership team for their work and guidance during the review process, in particular Stacey Aggabao MBA, MSN RN NEA-BC CEN CTACC; Elena Soriano CLS, MLS (ASCP); Susan Tannenbaum CLS (ASCP); and Kathy M. Taylor.
References
- Levinson Z, Godwin J, Neuman T. Hospital margins rebounded in 2023, but rural hospitals and those with high Medicaid shares were struggling more than others. KFF. Published December 18, 2024. Accessed June 24, 2025. https://www.kff.org/health-costs/issue-brief/hospital-margins-rebounded-in-2023-but-rural-hospitals-and-those-with-high-medicaid-shares-were-struggling-more-than-others/.
- Krishnamurthy B. Margin misconceptions: What do break-even hospital margins mean for patient care? American Hospital Association | AHA News. Published August 1, 2023. Accessed June 24, 2025. https://www.aha.org/news/blog/2023-08-01-margin-misconceptions-what-do-break-even-hospital-margins-mean-patient-care.
- Costs of caring. American Hospital Association. Published April 2025. Accessed June 24, 2025. https://www.aha.org/costsofcaring.
- Shaik T, Mahmood R, Kanagala SG, et al. Lab testing overload: a comprehensive analysis of overutilization in hospital-based settings. Proc (Bayl Univ Med Cent). 2024;37(2):312-316. doi:10.1080/08998280.2023.2288788.
To take the test online go HERE. For more information, visit the Continuing Education tab.
About the Author

Jaime A. Nieto Sierra, DBA, MHA
is a retired U.S. Air Force veteran and a published, award-winning, social sciences researcher with a rich array of skills and over 17 years of leadership experience in healthcare and life sciences administration. He currently serves as the Director of Operations for Ambulatory Services and Pathology General at Kaiser Permanente’s Greater Southern Alameda Area.
Prior to joining Kaiser, Jaime served in the U.S. Air Force, where he held leadership positions in various healthcare settings, including academic institutions, clinical lab and investigation facilities, and hospitals of varying sizes, including mid-sized and large facilities. His areas of expertise include business strategy, project management, organizational behavior, organizational learning, and regulatory compliance.

Apoorv Kumar
began his career in the heart of preventative medicine—vaccines—working with Bharat Biotech, one of India’s largest vaccine manufacturers. In this role, he led the commercialization of critical vaccines, including efforts to expand access to the company’s COVID-19 vaccine across global markets. His work included building out supply chains and forging long-term partnerships across Latin America, where he negotiated major contracts with multilateral agencies.
These experiences, though deeply impactful, revealed the immense pressure that infectious diseases place on an already strained health system. This realization spurred Apoorv to explore the healthcare provider segment and its operational challenges more closely. Through consulting engagements with Marin Health and an internship with Kaiser Permanente, he immersed himself in the strategic and financial decision-making processes of health systems. Currently pursuing a dual MBA/MPH at the University of California, Berkeley, Apoorv brings a unique lens that bridges biopharma innovation, public health, and hospital operations. With a foundation in chemical engineering, he is passionate about ensuring that high-quality healthcare can be delivered as efficiently as possible to as many people as possible.