The absence of a safety culture; What contributes to laboratory incidents?
To take the test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Describe the correlation of laboratory accidents, errors, and mistakes.
2. Discuss the importance of standard operating procedures that emphasize laboratory safety.
3. Identify physical environment (PE) categories that should be considered in PE rounds.
4. Describe how to incorporate laboratory safety into training and list effective management oversight tools to review safety indicators and assess staff safety competency.
There are different types of errors that occur in the clinical laboratory. Some are based around quality assurance, while others are related to safety. The goal when reducing each of these types of errors is to not only eliminate the potential harm to patients, but to the laboratory staff as well. Laboratory errors, mistakes, and accidents are interconnected (see Table 1), and one can be the cause of another. Laboratory accidents, for instance, are the result of staff errors or mistakes. To reach a goal of reducing laboratory accidents, you must first set out to reduce the occurrence of the errors and mistakes that lead to them.
In order to accomplish this, applying a proactive approach to laboratory safety is important. Staff education, physical environment surveys, initial and annual training, and especially management oversight all contribute to a reduction of laboratory errors, mistakes, and accidents while helping to grow a culture of laboratory safety. All four of these aspects of laboratory safety require a commitment to safety from laboratory leaders as well as cooperation between those leaders and laboratory staff. To truly excel in the creation of and maintenance of a laboratory safety culture, equal and active participation of lab management and lab staff is required.1
Standard operating procedures
Standard operating procedures (SOPs) are written to ensure that proper steps are taken to perform a task correctly and safely. In addition to ensuring accuracy, SOPs also increase the precision around task and test performance. This not only reduces variability, but also lowers the inherent risk involved with the procedure.
Policies and procedures offer a good opportunity to emphasize lab safety precautions and the tools available to further lower the inherent risks involved with laboratory analysis. Therefore, when given the opportunity, it is essential to incorporate safety when drafting or revising your SOPs. When safety steps or tools are written into a procedure, the author should be as specific as possible. For example, the step “don essential personal protective equipment” is quite vague and could be interpreted in several ways. Rather, specific wording such as “don a face shield and cryogenic gloves” would be much less subjective when working with an extreme cold hazard such as liquid nitrogen. Reducing subjectivity within laboratory actions will reduce the potential for mistakes while concurrently strengthening your safety culture.
Where safety-related information is placed within a document could also have an effect in its interpretation. Listing required safety items in sections at the beginning or at the end of an SOP may seem intuitive, but it may have a greater impact if incorporated into the procedure as discreet actions. If a safety action is required to perform a task, the SOP should include a prior step instructing the user to retrieve or utilize the safety device before moving forward. This gives the user a chance to pause and perform the safety action prior to being exposed to a hazard or an unsafe act.
The frequency and process of reviewing your SOPs can also impact your safety culture. The College of American Pathologists (CAP) requires the laboratory director or designee to review all technical policies and procedures at least every two years. This is the minimum requisite, and additional revisions should be made when new equipment is introduced to the lab or when processes change that affect how lab staff perform their duties. The Occupational Safety and Health Administration (OSHA) has more stringent requirements when it comes to safety and recommends laboratories review SOPs related to certain safety regulations. For example, procedures related to hazardous chemicals must be reviewed annually to certify that they are accurate and current (29 CFR 1910.119(f)(3)).2 Again, these are minimum requirements that should be reviewed further anytime the processes in the lab are altered.
Additionally, increasing the number of individuals who review the SOP can also offer a different perspective on the documents and creates the potential to catch errors and missing information. Laboratory managers, subject matter experts, and technical staff who utilize these procedures on a regular basis should all be included in the review process, and their input should be requested.
Physical environment rounding
Monitoring the laboratory’s physical environment can preemptively resolve safety issues that might lead to accidents and errors, and the practice can help others develop good housekeeping awareness to reduce accidents. Hospital physical environment (PE) rounds, sometimes referred to as environment of care (EOC) rounds, should be conducted often to maintain a safe and functioning facility environment. However, the areas of the hospital or lab that require PE rounding, and the frequency of evaluations, are not always clearly defined. The determination may be dependent on the laboratory or hospital accrediting agency. For example, Centers for Medicare & Medicaid Services (CMS) regulations (42 CFR 482.41) state that “the overall hospital environment must be developed and maintained in such a manner that the safety and well-being of patients are assured,” leaving the individual facility to determine the frequency of PE Rounds.3 Other hospital accrediting agencies, like Det Norske Veritas (DNV) refer to the National Integrated Accreditation for Healthcare Organizations (NIAHO) standard PE.3 SR.5, which states that “the Safety Management System shall require periodic surveillance of the hospital grounds to observe and correct safety issues that may be identified.”4 Some accrediting agencies have different requirements for patient areas and non-patient areas. The Joint Commission (TJC) requires environmental rounding in patient areas that must occur every six months and annually in non-patient areas (TJC standards EC.04.01.01, EP 12 and EC.04.01.01, EP 13 respectfully).5
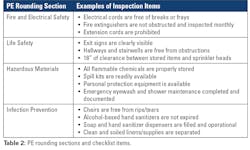
Physical environment monitoring checklists can be broken up into different categories and can include sections such as fire and electrical safety, life safety, hazardous waste, and infection prevention (see Table 2). Additional sections can include staff knowledge and emergency management. Upon completion of rounding, the survey should be reviewed to evaluate potential safety issues, and if found, resolved swiftly to eliminate any risk to employee safety.Training
Inadequate training can result in undesired outcomes across all three phases of laboratory testing: pre-analytical, analytical, and post-analytical. New hire and initial training sessions are your first chances to introduce your learners to new material, so first impressions are very important. In addition, emphasizing safety from the beginning of employment can help build and strengthen your laboratory’s safety culture. Ongoing training and monitoring are also critical and will be essential components of your education program as a means to reduce laboratory errors and accidents.6 Some of the greatest barriers to maintaining a safe lab environment include a lack of training and availability of training material as references when needed.7
General new hire training can include topics such as bloodborne pathogens and chemical hygiene training, but safety learning does not stop there. When reviewing SOPs initially, it is important to concentrate on the safety equipment and processes associated with safety found in SOPs. For example, the OSHA Formaldehyde standard states that formaldehyde training should include topics such as dangers associated with working with the hazardous chemical, exposure limits and monitoring requirements, signs and symptoms of exposure, correct spill responses, how to use the required PPE, and what to do in emergency situations (OSHA Standard 1910.1048).8 Users should be able to speak to similar points before being able to work independently with similar hazardous materials.
PPE training should not just include enforcement of use, it should cover the reasons why certain PPE is necessary in order to help educate the employee to best understand the need. According to the OSHA standard regarding PPE training, in addition to knowing which protective equipment to use, employees are required to know the reason PPE is required for a particular task, how to properly don and doff the PPE, and even to understand the maintenance and limitations of the equipment.9
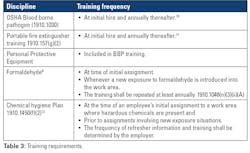
As with SOPs, the frequency of staff training can be regulated by your accrediting agency. Many different training modules and/or classes are required at initial hire and annually thereafter. These may include bloodborne pathogen training, fire and electrical training, and hazardous waste management.
In a research paper published by the National Bureau of Economic Research, it is established that maintaining a strong safety culture does not have a negative impact on the productivity of academic research labs. The impetus for the study was the "crackdown" on research lab safety after the accidental death of a California student in 2008, but the information in the study can be applied to clinical labs as well. It is unfortunate that injuries and even death had to force better safety oversight and practices in these labs, but it is good to prove that prioritizing lab safety and providing adequate training is now known not to hamper the work done in the laboratory setting.Management oversight
The continuous monitoring of safety issues and the timely response to incidents is key to reducing accidents and errors. Lab management has the responsibility of ensuring a safe environment for staff and addressing safety concerns brought to their attention.
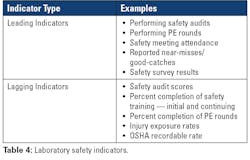
One method of monitoring lab safety is through evaluating safety indicators (see Table 4). By utilizing safety indicators, laboratory leadership can assess the effectiveness of their safety culture and reduce any potential hazards. Managers can identify safety issues before an accident occurs through leading indicators while simultaneously evaluating the effectiveness of their safety program through lagging indicators. This information is not useful, of course, if it is not analyzed and acted upon. As with other performance indicators, managers should include a review of the data collected as part of their performance monitoring system and make adjustments to improve indicator scores such as injury rates and safety audit scores.In addition to indicators, laboratory managers must also monitor staff performance and compliance with safety policies and procedures. Laboratory leaders should hold staff accountable for unsafe practices and behavior in the lab. Lack of oversight can lead to poor quality of work and potentially increased errors and accidents.
A challenge to holding staff accountable for safety in the lab is the perceived power distance that may exist amongst the different positions of employees. In some environments, those responsible for safety may be apprehensive about coaching someone in a perceived higher position (i.e., manager, medical director) about a safety issue. Those in “higher” roles might choose to ignore certain safety requirements, which can lead to the occurrence of errors and accidents.14 Power distances should be lowered or eliminated in a laboratory setting since it is important that safety applies to all in the department.
One method of holding staff accountable regarding safety is to include safety in the lab annual competency assessments. The College of American Pathologist lists six requirements to assess the competency of laboratory employees in the various sections of the lab. A helpful addition to these requirements would be an element of laboratory safety. It may be assumed that staff are conducting their daily operations in a safe manner, yet this is not assessed or documented, so it is unknown if deviation from laboratory safety policy occurs. Adding a safety component to initial and annual competency assessments allows for staff accountability once training is complete and on an ongoing basis. In addition to ongoing monitoring, an annual assessment of staff safety practices provides staff with awareness that safety is a focus for lab leadership, and it is important to the organization.
Summary
There are several factors that can contribute to laboratory errors and accidents as a result of staff mistakes. Understanding the safety hazards in the lab and how to mitigate the associated risks, in addition to timely and effective training, the monitoring of the physical environment, and manager engagement are tools that can aid in the reduction of safety incidents. These four components of laboratory operations help contribute to the overall safety culture of the workplace by making staff and laboratory leadership aware of the dangers they may encounter and by preventing these undesired events.
References
1. National Research Council (US). Prudent Practices in the Laboratory: Handling and Management of Chemical Hazards: Updated Version. Washington (DC): National Academies Press (US); 2011. 1, The Culture of Laboratory Safety. Available from: https://www.ncbi.nlm.nih.gov/books/NBK55882/.
2. Osha.gov. Accessed September 26, 2023. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.119.
3. Ecfr.gov. Accessed September 26, 2023. https://www.ecfr.gov/current/title-42/chapter-IV/subchapter-G/part-482/subpart-C/section-482.41.
4. National integrated accreditation for healthcare organizations (niaho ® ). Dnv.us. Accessed September 26, 2023. https://www.dnv.us/Images/DNV%20GL%20NIAHO%20-%20HOSPITAL%20-%20PROPOSED%20Rev%2011-1%20-%20PE%20Excerpt%20-%2010-25-2016_tcm14-79671.pdf.
5. Patient Safety Systems (PS). Jointcommission.org. Accessed September 26, 2023. https://www.jointcommission.org/-/media/tjc/documents/standards/ps-chapters/camh_04a_ps_all_current.pdf.
6. Clemente M. Evaluation of a Training Program through the Analysis of Occupational Accidents in a Health Care Center. Revista Enfermagem UERJ. 2003;11(1):34-38.
7. Alqeer A, Alo E. Improved Safety Practices in Teaching Laboratories of Health Institute. International Journal of Vocational Education and Training Research. 2022;8(1):6-11. doi:10.11648/j.ijvetr.20220801.12.
8. Osha.gov. Accessed September 26, 2023. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1048.
9. Osha.gov. Accessed September 26, 2023. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.132.
10. Osha.gov. Accessed September 26, 2023. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1030.
11. Osha.gov. Accessed September 26, 2023. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.157.
12. Osha.gov. Accessed September 26, 2023. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1450
13. Galasso A, Luo H, Zhu B. Laboratory safety and research productivity. Res Policy. 2023;52(8):104827. doi:10.1016/j.respol.2023.104827
14. Basbug G, Cavicchi A, Silbey SS. Rank has its privileges: Explaining why laboratory safety is a persistent challenge. J Bus Ethics. 2023;184(3):571-587. doi:10.1007/s10551-022-05169-z.
About the Author

Jason P. Nagy, PhD, MLS (ASCP)CM,QLS
is the Laboratory Safety Support Coordinator at Sentara Health, a multi-hospital system in Virgina and North Carolina. Jason brings almost 20 years of laboratory experience to the lab as a medical laboratory scientist (MLS) and more recently in laboratory safety and education roles.