How lab diagnostics can support COVID-19 patient management
For a printable version of the January CE Story and test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Provide an overview of coronavirus disease 2019 (COVID-19) and the underlying pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
2. Recall COVID-19 etiology and its risk factors.
3. Demostrate a working knowledge of the clinical course of COVID-19.
4. Describe key biomarkers and their relevance in COVID-19 clinical decision-making, with focus on IL-6, PCT, D-dimer, cardiac troponin, NT-proBNP and ferritin.
As the coronavirus disease 2019 (COVID-19) pandemic continues to burden the healthcare system with increased demand for bed capacity and personal protective equipment (PPE), as well as a heightened focus on safeguarding the health of other patients and hospital staff and the overall reprioritization of resources, the clinical laboratory has been meeting a parallel set of challenges. The initial reduction in overall testing volume and revenue, driven by fewer primary care visits and reduction of elective surgery, was quickly overtaken by escalating demand for COVID-19 testing and reagents, especially for PCR testing. As overall testing volume continues to return to near normal, new opportunities for labs to contribute to the care of COVID-19 patients are emerging. The scientific community’s response to COVID-19 has resulted in a large volume of publications as scientists and clinicians share their experience and glean new insights. For example, it is estimated that hospitalization is required in about 20 percent of diagnosed cases, with 5 percent requiring intensive care unit (ICU) admission and/or respiratory support.1 Significantly, for COVID-19 patients, median length of stay is well above average: 10.1 days for all admissions and 10.6 days for the intensive care unit (ICU). At the same time, the expanding knowledge base about the clinical course of COVID-19 now includes data correlating levels of biomarkers with patient status to support clinical decision making.2 To date, the FDA has granted emergency use authorization (EUA) status to two commercial assays for interleukin-6 (IL-6) to assist in identifying severe inflammatory response in COVID-19 patients, adding to the diagnostic tests available to help clinicians make patient care decisions. This article provides an overview of IL-6, procalcitonin (PCT), D-dimer, cardiac troponin, N-terminal pro B-type natriuretic peptide (NT-proBNP) and ferritin and their potential clinical significance in critically ill patients.
COVID-19 etiology and risk factors
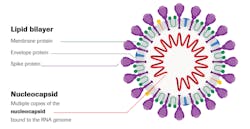
Coronavirus disease (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a large, enveloped RNA virus.3 The lipid bilayer envelope, membrane proteins and nucleocapsid protect the virus when it is outside the host cell. Genetic analyses indicate SARS-CoV-2 originated in bats and is analogous to SARS-CoV, the coronavirus that emerged in 2003–2004 and caused epidemic disease, and Middle East respiratory syndrome coronavirus (MERS-CoV).
Disease progression
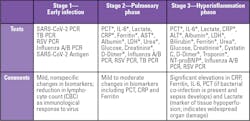
Figure 2 summarizes a three-stage classification system for COVID-19 illness, with three grades of increasing severity that correspond with distinct clinical findings, response to therapy and clinical outcomes.5
Stage 3: The hyperinflammation phase represents about 5 percent of diagnosed cases, characterized by the body responding to the infection and manifesting as an extrapulmonary systemic hyperinflammation syndrome. Clinical symptoms include acute respiratory distress syndrome (ARDS), heart failure, septic shock, coagulopathy, acute cardiac injury, acute kidney injury and secondary infections, all with worse outcomes.5,6 Inflammatory cytokines and biomarkers, such as IL-6, ferritin and D-dimer, are significantly elevated in these patients, as are troponin and N-terminal pro B-type natriuretic peptide (NT-proBNP).5 This article will focus on biomarkers and laboratory testing for this group of critically ill patients.
Laboratory diagnostics in COVID-19 patient management
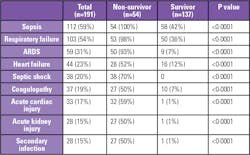
Laboratory testing protocols vary by institution. Commonly used tests by COVID-19 clinical staging are summarized in Table 1. Notably, many of the tests are markers of complications with poor survival, as summarized in Table 2.
Unique challenges in critical care testing
Critical care marks a turning point in disease progress. More than ever, clinicians are looking for answers to make life-saving decisions, decisions that can also impact health system resource allocation and prioritization. Test results are often needed urgently. Just as urgently, clinicians need to understand test results and how they translate into patient care decisions, especially as accelerated publication schedules continue to offer new data that can change clinical practice. The following is an overview of key biomarkers that can impact patient care decisions, with the goal of helping the clinical laboratory inform and educate their clinical care counterparts within the healthcare system.
Interleukin-6 (IL-6)
Interleukin-6 (IL-6) is a marker of severe inflammatory response in COVID-19 patients and the abnormal release of circulating cytokines such as interferons, interleukins and tumor necrosis factors, cell-signaling molecules that play a key role in the immune response.6 Overproduction of early response proinflammatory cytokines results in a “cytokine storm” that leads to severe downstream effects with multi-organ involvement, including development and progression of acute respiratory distress syndrome (ARDS). Elevated circulating concentrations of IL-6, a key mediator of inflammation, can serve as an early alarm signal of SARS-CoV-2 infection-triggered hyperinflammation, helping clinicians identify this process in severely ill COVID-19 patients.7 Aside from assisting in the identification of hyperinflammation in COVID-19 patients, IL-6 may predict respiratory failure in hospitalized, symptomatic COVID-19 patients and thus provide an objective data point to assist clinicians in mechanical ventilation resource allocation. One study found that after IL-6 of 35 pg/mL was reached, the median time to mechanical ventilation was 2 days.8 Because IL-6 is released early during a severe infection, it can help physicians to identify severely ill COVID-19 patients in a timely manner.
Procalcitonin (PCT)
Procalcitonin (PCT) aids in measuring the inflammatory response to bacteria, enabling the differentiation of bacterial infections from nonbacterial causes of lower respiratory tract infections. PCT levels rise rapidly, generally within 3 to 6 hours after a bacterial infection. In COVID-19 patients, increased PCT is associated with higher risk of severe COVID-19 and may reflect bacterial co-infection.9 Increase in PCT concentration correlates with the severity of the bacterial infection, with significant elevated levels being associated with patients at risk of developing severe sepsis or septic shock, both of which have been identified as COVID-19 complications.11 (See Table 2.) Sustained elevated PCT is associated with patients with ARDS.10 For COVID-19 patients with respiratory failure requiring mechanical ventilation, empiric antimicrobial treatment may be initiated.12 Conversely, a decrease in PCT concentration signals relief of infection. In this context, PCT testing can guide de-escalation of antimicrobial therapy (e.g., shortening the duration of therapy or discontinuing empiric antibiotics) and support its optimal use — the core elements of antimicrobial stewardship.13
D-dimer
D-dimer is one of several fragments produced during the degradation of a fibrin clot by plasmin. Viral infections elicit a systemic inflammatory response and cause an imbalance between procoagulant and anticoagulant homeostatic mechanisms. The dysregulation of the coagulation cascade and the subsequent formation of intra-alveolar or systemic fibrin clots are prominent findings in coronavirus infections associated with severe respiratory diseases such as SARS-CoV-1 and MERS-CoV. The most common pattern of coagulopathy observed in patients hospitalized with COVID-19 is characterized by elevations in D-dimer and fibrinogen levels. As shown in Table 2, coagulopathy has been identified as a complication in COVID-19 patients. Another study of 560 hospitalized COVID-19 patients showed that 69.4 percent of the patients who were in the ICU, were on mechanical ventilation, or died had elevated D-dimer levels.14
Cardiac troponin and N-terminal pro B-type natriuretic peptide (NT-proBNP)
Cardiac troponin and N-terminal pro B-type natriuretic peptide (NT-proBNP) are relevant in COVID-19 because myocardial injury is prevalent among patients hospitalized with COVID-19, and patients with cardiovascular disease (CVD) are more likely to have myocardial injury than patients without CVD.15 One study showed that patients with underlying CVD accounted for 4.2 percent of COVID-19 cases but 18.3 percent of COVID-19 deaths.16 For critically ill COVID-19 patients, the utility of biomarkers such as cardiac troponin and NT-proBNP lies also in their high negative predictive value. When the values are low and stable, they provide the confidence needed to triage patients away from imaging or further work-up for cardiac-related issues.
Ferritin
Ferritin is an indicator of severe inflammation in COVID-19 patients and is associated with worse clinical outcomes.2,17 Several studies showed that serum ferritin level correlates with severity of disease. In COVID-19 patients who died, ferritin levels were high upon hospital admission and throughout the hospital stay.18
The clinical laboratory’s role
“While the trajectory of this outbreak is impossible to predict, effective response requires prompt action from the standpoint of classic public health strategies to the timely development and implementation of effective countermeasures.”3
As the COVID-19 pandemic continues, clinical laboratories can take this call to action to heart by sharing our knowledge and expertise with colleagues -- nurses, respiratory therapists, phlebotomists, administrators, clinicians — and begin a dialogue about the role of lab diagnostics in supporting COVID-19 patient care decisions, with the goal of improving patient outcomes.
References
- Auld SC, Caridi-Scheible M, Blum JM, et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med 2020 Sep;48(9): e799-e804. doi: 10.1097/CCM.0000000000004457.
- Thompson S, Bohn MK, Mancini N, et al. IFCC interim guidelines on biochemical/hematological monitoring of COVID-19 patients. Clin Chem Lab Med 2020;58(12). doi: 10.1515/cclm-2020-1414.
- Paules CI, Marston HD, Fauci AS. Coronavirus infections — more than just the common cold. JAMA. 2020;323(8)707-708. doi: 10.1001/jama.2020.0757.
- Jordan RE, Peymane A, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ 2020 Mar 26;368:m1198. doi: 10.1136/bmj.m1198.
- Siddiqi HK, Mehra MR. 2020. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant 2020 May;39(5):405-407. doi: 10.1016/j.healun.2020.03.012.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020 Mar;395(10229)1054-1062. doi: 10.1016/S0140-6736(20)30566-3.
- Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th Edition). http://kjfy.meetingchina.org/msite/news/show/cn/3337.html. Accessed December 8, 2020.
- Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol 2020; 146:128-136. doi: 10.1016/j.jaci.2020.05.008.
- Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta 2020 Jun; 505:190-191. doi: 10.1016/j.cca.2020.03.004.
- Liu Y, Sun W, Chen L, Wang Y, Zhang L, Yu L. Clinical characteristics and progression of 2019 novel coronavirus-infected patients concurrent acute respiratory distress syndrome. doi: 10.1101/2020.02.17.20024166. Note: This article is a preprint and has not been peer-reviewed.
- Schuetz P, Amin DN, Greenwald JL. Role of procalcitonin in managing adult patients with respiratory tract infections. Chest 2012 Apr;141(4):1063-1073. doi: 10.1378/chest.11-2430.
- Alhazzani W, Hylander Møller M, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med 2020 Jun;48(6): e440-e469. doi: 10.1097/ccm.0000000000004363.
- Centers for Disease Control and Prevention. Core elements of hospital antibiotic stewardship programs. https://www.cdc.gov/antibiotic-use/core-elements/hospital.html
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708-1720. doi: 10.1056/NEJMoa2002032.
- Lala A, Johnson KW, Januzzi JL, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 2020;76(5):533-546. doi: 10.1016/j.jacc.2020.06.007.
- Tan W, Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int J Cardio 2020 Jun 15; 309:70-77. doi: 10.1016/j.ijcard.2020.03.063.
- Zeng F, Huang Y, Guo Y, et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis 2020 Jul; 96:467-474. doi: 10.1016/j.ijid.2020.05.055.
- Vargas-Vargas M, Cortés-Rojo C. Ferritin levels and COVID-19. Rev Panam Salud Publica 2020; 44 :e72. doi: 10.26633/RPSP.2020.72. 11.
About the Author

Justin Jones, PhD
has more than a decade of experience in diagnostics and translational science, including leading assay development projects at Siemens Healthineers. In his current position as a Field Medical Partner, he specializes in cardiac biomarkers and clinical education. Justin holds a PhD in biochemistry and is dedicated to connecting laboratory insights with patient care.