Hyperglycemia associated with hospitalization for severe COVID-19 infection
Earning CEUs
For a printable version of the October CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
Passing scores of 70 percent or higher are eligible for 1 contact hour of P.A.C.E. credit.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Describe the factors associated with increased risk of death from COVID-19.
2. Describe the evidence showing an association between diabetes, prediabetes and obesity and severe COVID-19.
3. Recall a confluence of factors linking hyperglycemia and the ACE2 pathway with severe COVID-19 infection.
4. Discuss the different types of assays for measuring HbA1c.
In December 2019, the novel Coronavirus 2019, also known as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which causes COVID-19, began to rapidly spread around the globe. The total number of cases at this time is likely much greater than official diagnoses, as many may not know they are infected. Mild cases may be asymptomatic or experience vague symptoms, which patients do not consider severe enough to warrant testing, but these cases are still capable of transferring the virus to others, for whom the disease may be severe or even deadly.
This highlights the necessity to find factors to determine the risk stratification of patients presenting with COVID-19 infections. This article will focus primarily on the risk factors of diabetes mellitus (DM) and uncontrolled hyperglycemia. It is important to acknowledge that diabetes is linked to comorbidities (such cardiovascular disease, renal insufficiency, and obesity) that are also risk factors for poor COVID-19 outcomes. There is growing evidence that the systemic pro-inflammatory state engendered by uncontrolled or poorly controlled diabetes and the related upregulation of Angiotensin Converting Enzyme II may be behind the increased severity of COVID-19 infections in these patients.
Once molecular testing for COVID-19 gave a more precise picture of the pandemic, it became clear that a subset of infected patients developed acute respiratory distress (ARDS), requiring hospitalization. Early reports from the outbreak in China identified the increased risk of death in these hospitalized patients,1 and identified risk factors for hospitalization, including people over the age of 65, males, and those with comorbidities such as hypertension, diabetes, cardiovascular diseases and respiratory diseases. Subsequent publications included other population groups, including one that analyzed the health records of over 17 million individuals in Great Britain, and similarly found comorbidities associated with hospitalization and increased risk2 of severe COVID-19 to include cardiovascular, respiratory, kidney, neurological, or liver disease, history of hematological malignancy or other recent cancer, autoimmune conditions, obesity, and diabetes. (The study also found that Asian and Black demographics are at increased risk of death, which was only partially attributed to comorbidity, deprivation, or other risk factors.)
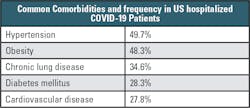
A U.S. study (compiled by the COVID-19 Associated Hospitalization Surveillance Network) similarly highlighted the importance of comorbidities in hospitalized US COVID-19 patients as summarized in Table 1.
Hyperglycemia
Among people with COVID-19 who require hospitalization, studies have found that many also have diabetes. For example, a recently published study involving 184 patients admitted to a New Jersey hospital for COVID-19 found that 85.9 percent had either diabetes mellitus (62.0 percent, HbA1c ≥6.5 percent) or prediabetes (23.9 percent, HbA1c =5.7-6.4 percent).3 A further 4.3 percent of patients had a normal HbA1c, but a BMI >30 kg/m.2
The prevalence of diabetes in this patient group is 4.7 times higher than that of the general U.S. population, while the prevalence of prediabetes was 1.3 to 6.4 times higher. A significant number of patients were clinically obese, with a BMI > 30 kg/m2. Presumably, diabetic and non-diabetic individuals are as likely to be exposed to COVID-19, but some aspect of diabetes results in diabetics more frequently experiencing poor outcomes and requiring hospitalization.
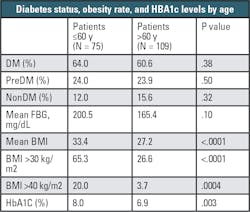
The study also found that HbA1c levels measured at admission, taken as an indication of glycemic control over the prior nine to 12 weeks, were in the prediabetic range in 37.4 percent of patients, and in the range of a diabetes diagnosis in 48.0 percent of patients.3 As outlined in Table 2, when sorted by age, COVID-19 patients 60 years old or younger were more likely to be clinically obese (65.3 percent) compared to those patients older than 60 years (26.6 percent). Similarly, COVID-19 patients 60 years old or younger had a significantly higher mean HbA1c than older patients (8.0 percent HbA1c vs 6.9 percent HbA1c, P=.003), suggesting more pronounced metabolic dysregulation in the younger hospitalized patients. Whereas the older hospitalized COVID-19 patients likely had many additional comorbidities, among the younger patients it appears that diabetes – and not just diabetes but uncontrolled or poorly controlled diabetes – is contributing to poor outcomes from the COVID-19 infection.
Similar to initial hospitalization, the need for intubation can be taken as a further indicator of severity and progression of COVID-19. The data about intubation continues to support the importance of glucose metabolism dysregulation in predicting COVID-19 severity. In the population of patients requiring intubation, 97.6 percent were either known diabetics or had elevated HbA1c, with the mean HbA1c being higher in the intubated group (8 percent HbA1c) than in the non-intubated group (7.2 percent HbA1c). Similarly, fasting blood glucose (FBG) at admission was elevated in the intubated group (238.0 vs. 163.7 mg/dl), and only one patient in the intubated group was categorized as non-diabetic, although in that case the patient was clinically obese. Some patients in the study population died without being intubated, and the majority were diabetic (70.9 percent), or prediabetic (16.7 percent).
It should be noted that both pre-existing stress and the use of corticosteroids can cause increases in blood glucose, especially in severely ill hospitalized patients, which may complicate the interpretation of this data. However, of the 54 patients in this study classified as prediabetic, almost half had markedly elevated fasting blood glucose levels in the absence of glucocorticoid therapy. Additionally, six patients without known diabetes mellitus and a normal HbA1c on admission had repeatedly elevated fasting blood glucose levels.
In summary, all of these findings present a link between dysregulation of glucose metabolism and severe COVID-19 infections in a hospitalized population.3 Hyperglycemia and obesity can be used to stratify the risk of an adverse event (requiring intubation or resulting in death).
Other lab abnormalities in hospitalized COVID-19 patients
What other laboratory assays could be useful in stratifying the risk of patients admitted to the hospital for COVID-19 infection? With severe COVID-19 infection, abnormal laboratory results may be caused by systemic immune damage from the inflammatory response. However, when measuring the immune response, many of the markers detectable by laboratory assay (such as elevation of: aspartate aminotransferase, serum creatinine, troponin, procalcitonion, lactate dehydrogenase, GGT) cannot be used to predict those patients that would require intubation or who would ultimately expire.
More interesting are the noted increases in the levels of inflammatory-related markers – such as C-reactive protein, serum ferritin, Erythrocyte sedimentation rate (ESR), Interleukin-6, and coagulation parameters such as D-dimer and Fibrinogen – that may be associated with the risk for severe COVID-19.4
These are interesting in the context of diabetes because type 2 diabetes is widely reported as either a chronic, low-grade inflammatory disease caused by long-term immune imbalance, or a metabolic syndrome associated with obesity. In the latter case, obesity is also linked to chronic inflammation, characterized by an increased abundance and activation of innate and adaptive immunity cells in adipose tissue, along with an increased release of inflammatory factors and chemokines both locally and systemically.5 This connection draws a direct link between the increased risk of severe COVID-19 outcomes and chronic inflammation.
Scientists have independently hypothesized that COVID-19 induces a cytokine storm similar to that described in SARS (the viral outbreak first identified 2003). Type 2 diabetics – who would be more susceptible to the multi-organ failure resulting from such a cytokine storm due to their underlying chronic inflammation – would then be at greater risk of severe outcomes from COVID-19. This aligns well with the observations presented in the study above. Further, glucose dysregulation may lead to more severe COVID-19 infections by another mechanism, this time involving the entry of COVID-19 into host cells.
Angiotensin Converting Enzyme 2 (the cellular portal for COVID-19)
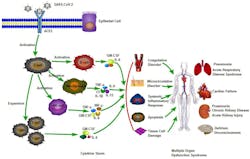
Of particular interest to COVID-19 infection is Angiotension Converting Enzyme 2, or ACE2. This enzyme – found in many organs including the lungs, heart, blood vessels, kidneys, liver, and GI tract – is part of the renin-angiotensin-aldosterone pathway, and plays a key role in regulation of blood pressure, wound healing and inflammation.
ACE2 acts in the body to convert Angiotensin II to inactive forms, which modulates the effects of this molecule responsible for increasing blood pressure and inflammation. Angiotensin II has, in particular, been linked to damage to blood vessel linings and various forms of tissue damage.
The severity of a particular COVID‐19 infection could be explained by the factors influencing this pathway. Not only increased concentration of glycosylated SARS‐CoV‐2 viral particles in the lung epithelium, but increased concentration of glycosylated (reduced activity) ACE2 receptors in the lung epithelium may influence the degree and control of the pulmonary immune response to the SARS‐CoV‐2 spike protein at the key time frame of approximately day eight to 10 after symptom onset.
This is because, beyond ACE2’s involvement in the inflammatory cascade, it appears to be the primary receptor for entry of SARS‐CoV‐2 into various epithelial tissues. (As a side effect, due to the high concentration of ACE2 in the tongue and oropharynx, infection by COVID-19 can be associated with loss of taste and smell during the acute infection.) The mechanism of viral entry into ACE2-containing cells is through an envelope-anchored spike protein. This allows entry of coronaviruses into host cells by binding to a host receptor and fusing viral and host membranes. Scientists believe that increases in glycosylation of both the viral spike protein, as well as ACE2 can enhance viral binding to ACE2 and the degree of the resultant immune response to the virus. This increased glycosylation is directly related to the patient’s blood glucose level over the 10-12 days before the severe COVID-19 symptoms appear.6
Here we have found a confluence of factors linking hyperglycemia and the ACE2 pathway with severe COVID-19 infection. Not every patient presenting with hyperglycemia associated with COVID-19 has a history of diabetes mellitus, and many have a normal HbA1c on presentation. Instead, entirely apart from whether there is a prior history of diabetes, high fasting plasma glucose is an independent risk factor for morbidity and mortality. For those patients without prior diabetes and without steroid administration, hyperglycemia may result from initial viral infection of the pancreas and lungs, with loss of function of the endocrine (beta) cells of the pancreas, and resultant hyperglycemia.
This hyperglycemia results in upregulation of glycosylated ACE2 in the lungs, and further enhancement of viral binding to these glycosylated receptors. Notably, the glycosylated ACE2-receptors used as entry points by COVID-19 are then incapable of performing their regulatory action on Angiotensin II, further disabling the control of the inflammatory pathway.
As viral binding is thought to be enhanced by glycosylation of the ACE2 receptor, it is likely that the amount of glycosylated ACE2 receptor, and not simply the amount of ACE2 alone, is responsible for increased virus binding and fusion. If true, this argues for better glycemic control in patients with prediabetes and diabetes as a potential mechanism to slow COVID‐19 spread and reduce the severity of symptoms, as better glycemic control should promote cellular pathways to downregulate the glycosylation of ACE2 receptors. Additionally, since 3.8 percent of the American population without a history of diabetes or prediabetes has a hemoglobin A1c over 6.1 percent in random sampling, use of high A1c as a risk stratification for COVID‐19 could have merit.6
The use of HbA1c in the COVID-19 pandemic
Given the evidence suggesting that diabetes, prediabetes or obesity are risk factors for severe COVID-19, HbA1c is an important test for patients with suspected or confirmed SARS-CoV-2 infection. The evidence also supports rigorous screening of non-COVID-19 patients for diabetes or prediabetes because this will help providers know which patients would benefit from lifestyle changes that would lower their risk for severe COVID-19 should they become infected.
The groups of patients who would benefit from HbA1c testing are:
- Patients with symptomatic COVID-19 infection. Many patients with respiratory distress due to COVID-19 have HbA1c included in their admission laboratory orders. In patients with hyperglycemia, an increased HbA1c would indicate that the patient has had an increased glucose for a time predating the acute viral infection, and that these patients should be classified as unrecognized diabetics now infected by COVID-19, with the highest risk of complications from the infection. Patients presenting with HbA1c between 5.7-6.4 percent and hyperglycemia, are prediabetic, with an intermediate risk. Patients with hyperglycemia and a normal HbA1c would be non-diabetic patients presenting with high blood sugar, and with a lower associated risk of morbidity and mortality. Note that patients from the latter two categories (prediabetic and normal HbA1c with hyperglycemia) should have periodic HbA1c monitoring after discharge to detect progression to diabetes mellitus after recovery from the COVID-19 infection.
- Asymptomatic patient populations. This screening would be undertaken to detect patients in the prediabetic or unrecognized diabetic categories. Patients identified as either prediabetic or diabetic would benefit from lifestyle changes designed to reduce body mass index through enhanced exercise and dietary improvements. They could also be screened for associated comorbidities associated with adverse outcomes. Patients who subsequently become symptomatic for COVID-19 would already be aware of their diabetic state and whether they are at increased risk for a severe disease course.7
Further benefits may be gleaned from HbA1c screening of both symptomatic and asymptomatic populations. Some methods employed for the measurement of HbA1c, incidentally detect hemoglobin variants and thalassemias in patient populations. In particular, the detection of Hemoglobin S (alone or in combination with other common Hb variants D,C,E or thalassemia) is of great importance, as the Centers for Disease Control and Prevention (CDC) has issued guidance that individuals with sickle cell anemia or thalassemias are at increased risk of developing severe COVID-19 infection.8
For a patient presenting with acute respiratory compromise and who will have HbA1c testing done, it is also important to consider the testing environment. At presentation, the HbA1c testing will likely be performed without access to prior laboratory records. Screening of asymptomatic populations that have no specific history of diabetes mellitus will also likely be reviewed in isolation. In these settings, the American Diabetes Association (ADA) has suggested that the method chosen for HbA1c testing allow for the determination of a clinical condition that may affect the red cell circulating lifespan, such as homozygous or doubly-heterozygous hemoglobinopathies or thalassemias.9
Why is this important? The HbA1c assay is used as an estimate of the average glucose concentration (AGC) over the past nine to 12 weeks. The proportional relationship between HbA1c and AGC assumes that the patient’s hemoglobin molecules are exposed to glucose over a normal red blood cell (RBC) lifespan. Conditions that reduce the survival of circulating RBC’s will reduce the patient’s HbA1c in relation to the degree of reduction in red cell survival. Therefore, the detection of some of the more common causes of decreased (or increased) RBC survival would be important in determining whether the HbA1c level was an accurate reflection of a patient’s level of glycemic control.
Hemoglobinopathies or thalassemias may be clinically silent but may cause reductions in red cell circulation time. The ADA recommendation is key to correctly identify those patients who are without proper glycemic control, but where the condition is incidentally concealed by the patient’s hemoglobinopathy. In the current situation, we are similarly concerned with correctly spotting a COVID-19 patient with a significant comorbidity that may be concealed by a hemoglobinopathy, and also incidentally discovering the hemoglobinopathy, which itself may be a risk factor for severe COVID-19 infection.
Methods of analyzing HbA1c fall into two categories:
- Separation methods, such as high-performance liquid chromatography (HPLC) and capillary electrophoresis. These methods may incidentally detect many hemoglobinopathies and thalassemias for later confirmation.
- Non-separation methods, such as immunoassay, enzymatic cleavage, or boronate affinity chromatography. These are incapable of noting when hemoglobin variants or thalassemias are present, but they can be used if the patient’s prior results do not indicate the presence of a hemoglobinopathy or other disease state that can alter the RBC lifespan.
Clearly, methods capable of detecting (and appropriately flagging) the presence of abnormal hemoglobin molecules would allow the performing laboratory to notify the ordering clinician of an abnormality in the HbA1c trace. The clinician would then know that the reported HbA1c value may not reflect the patient’s average glucose concentration over the past 12 weeks. A falsely low HbA1c, if unrecognized, could classify a diabetic patient as being normo-glycemic in error.
It is advisable that the method chosen for screening give information related to the presence of hemoglobin abnormalities, and that with homozygous or double heterozygous conditions, an alternate test be chosen for screening.9 This is particularly important when testing acutely ill patients presenting with acute respiratory distress when no prior medical chart is available, or in screening programs looking for prediabetic or previously unknown diabetic patients in the general population in an effort to identify at-risk individuals.
Summary
Early in the COVID-19 pandemic, it was recognized that certain subsets of hospitalized patients had poorer outcomes, based on patient age, body mass index, and comorbidities. This review focused on the presence of hyperglycemia as an independent risk factor. The role of glycation of the ACE2 receptor as an enabler of COVID-19 binding, and the increased risk of immunologic storm due to underlying chronic inflammatory state was discussed. Expanding upon this connection, HbA1c testing at hospital presentation can be used for risk stratification of hyperglycemic patients.
Additionally, the authors suggest screening of asymptomatic patients to detect prediabetes and previously unknown diabetes mellitus, which could be useful in intiating lifestyle changes to reduce these patients’ risk of adverse events related to COVID-19. The choice of method for HbA1c screening should allow for an estimation of conditions that could artificially lower the HbA1c value, which could result in prediabetics or unknown diabetics being falsely classified as euglycemic.
References
- Zeng et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020 Aug; 81(2), e16-e25. (2020). doi: 10.1016/j.jinf.2020.04.021
- Williamson et al. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adjult NHS patients. 1-21. medRxiv. https://doi.org/10.1101/2020.05.06.20092999.
- Guo et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 March. epub ahead of print; e3319. doi: 10.1002/dmrr.3319
- Smith et al. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID-19. J Med Virol. 2020. epub ahead of print; 1-7. doi: 10.1002/jmv.26227
- Meshkani R, Vakill S. Tissue resident macrophages: key players in the pathogenesis of type 2 diabetes and its complications. Clin Chim Acta. 2016; 462:77-89. doi: 10.1016/j.cca.2016.08.015
- Brufsky A. Hyperglycemia, hydroxychloroquine, and the COVID-19 pandemic. J Med Virol. 2020; 92:770-775.
- Lohmann, T. The impact of red blood cell lifespan on HbA1c measurement. Medical Laboratory Observer, June 24, 2019.
- Coronavirus Disease 2019 (COVID-19): people with certain medical conditions. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed September 25, 2020.
- Diabetes Care. American Diabetes Association. Standards of Medical Care in Diabetes – 2020. January 2020. Volume 43, Supplement 1. https://care.diabetesjournals.org/content/43/Supplement_1 . Accessed September 24, 2020.
About the Author

Thomas Lohmann, MD
Serves as Director of Medical and Scientific Affairs for Sebia-USA. He is board certified in Anatomic and Clinical Pathology, and has 40 years of experience in Laboratory Medicine.

Matthew C. Wagner PhD
received his PhD in Chemical Engineering in 2006 from Georgia Institute of Technology. Since transitioning to industry, he works as a Scientific Affairs Specialist for Sebia Electrophoresis, provider of capillary and gel electrophoresis assays, including Capillary Hemoglobin A1c.