Assay addresses M genitalium diagnostic abilities
Earning CEUs
For a printable version of the May CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Recognize the historical facts and symptoms of M. genitalium.
2. Recall underlying conditions and complications that develop as a result of M. genitalium in men and women.
3. Recall difficulties in the detection and diagnosis of M. genitalium.
4. Discuss the AMES study in terms of prevalence, detection and findings.
Sexually transmitted infections (STI) with the bacteria Mycoplasma genitalium are a generally under-recognized health concern due to inherent difficulties in detecting the organism.1 Historical estimates of M genitalium prevalence in men and women in population-based surveillance studies from Australia, Scandinavia, the United Kingdom and United States range from 1 percent to 3 percent.2-5 Development of a highly sensitive nucleic acid amplification test (NAAT) enabled a large U.S. multicenter study to demonstrate overall M genitalium prevalence rates of 16.1 percent in women and 17.2 percent in men, suggesting that infection rates were much higher in populations attending STI and other medical clinics.6
In that study, M genitalium prevalence was reported to be higher in symptomatic vs asymptomatic subjects (women, 21.1 percent vs 7.5 percent; men, 19.3 percent vs 15.4 percent). Infection rates are also elevated in high-risk populations such as patients presenting to sexual health clinics or men with nongonococcal urethritis.7 M genitalium is now understood to be one of the most common causes of STIs such that in 2015, the Centers for Disease Control and Prevention (CDC) declared M genitalium STIs to be an emerging health concern.8-11
The role of Mycoplasma genitaliumAlthough M genitalium infections can also be found in the respiratory tract, the most clinically important infections are sexually transmitted and occur in the urogenital tract, causing urethritis in men and cervicitis in women.12,13 Untreated or persistent M genitalium infections have been reported to cause complications in men such as reactive arthritis and epididymoorchitis.14 Studies have reported complications of M genitalium infections in women including nongonococcal urethritis, bacterial vaginosis and vaginitis, endometritis, salpingitis and pelvic inflammatory disease (PID). M genitalium, as detected by NAAT technology, has been frequently reported among women with PID, with infection rates ranging from 13 percent to 16 percent.15,16
Among 125 women diagnosed with acute PID in the ACE Trial, a randomized double-blind study evaluating therapy for acute PID, 22 percent (n = 27) tested positive for M genitalium, whereas C trachomatis (CT), N gonorrhoeae (NG) and bacterial vaginosis were present in 14 percent, 7 percent, and 54 percent of women with acute PID, respectively.17 These results illustrate the important role that M genitalium tentatively plays in the pathogenesis of PID, and suggest that M genitalium may possibly be an underlying cause of infertility, preterm delivery and spontaneous abortion observed in women with PID.6,18,19
In a study where C trachomatis and N gonorrhoeae infections in women were accompanied by M genitalium infection, the authors concluded that C trachomatis and N gonorrhoeae infection increased the risk for M genitalium infection.7 Infection with M genitalium has been demonstrated to increase the risk for HIV infection and transmission, as well as infections with high-risk human papilloma virus (HPV).20
Distinguishing Mycoplasma genitalium infections from other STIs
As with STIs in general, M genitalium infections are predominantly asymptomatic, contributing to a lack of clinical recognition.18 Among patients with symptomatic STI, symptoms of C trachomatis, N gonorrhoeae, gonococcal and M genitalium infections are difficult to differentiate, often leading to misdiagnosis of the specific underlying bacterial infection. A lack of testing and poor testing sensitivity has also impeded adequate diagnosis of M genitalium.1
First-line treatment for M genitalium infection is azithromycin; however, resistance of M genitalium to this antibiotic is growing. A dose of azithromycin to treat C trachomatis may be insufficient to clear M genitalium and may engender M genitalium resistance to this macrolide antibiotic.14 Worldwide M genitalium macrolide resistance rates are estimated at 30-100 percent, depending on the country.7
Accurate diagnosis of M genitalium and antibiotic susceptibility are imperative for appropriate and adequate treatment.1,21 The absence of Food and Drug Administration (FDA)-cleared diagnostic tests for M genitalium prior to 2019 prevented performance of routine testing, leading to missed diagnoses and inappropriately treated or untreated infections, which raised the risk for persistent infections with adverse outcomes.
FDA-cleared Mycoplasma genitalium assays – a recent step forward
Nonfeasibility of routine in vitro culturing for M genitalium detection has necessitated the use of more-sensitive NAATs to identify the bacteria. Use of an NAAT is now the CDC-recommended detection method for M genitalium.8 Despite the higher sensitivity of NAATs, M genitalium infections have low organism loads and each bacterium has only a single copy of the small M genitalium genome, which together limits DNA-based NAAT detection methods.13,21 Alternatively, each M genitalium bacterium contains 100–1,000 copies of 16S ribosomal RNA (rRNA), making this nucleic acid molecule an attractive amplification target for organism detection.
An FDA-cleared, transcription-mediated amplification (TMA) NAAT targets a specific region of the M genitalium 16S rRNA for amplification and subsequent detection.1 The assay has been validated against a composite reference standard for M genitalium using other TMA NAATs that amplify different regions of M genitalium rRNA.22 This FDA-cleared assay has been used in research settings to study the epidemiology of M genitalium1 and was recently clinically validated in the AMES Prospective Multicenter Clinical Study.23 The assay was the first FDA-cleared diagnostic test for M genitalium. Recently, another NAAT assay for the detection of M genitalium was approved for the cobas 6800 and 8800 systems from Roche Diagnostics.24
AMES prospective multicenter clinical study
The AMES Study was conducted to validate the performance of an investigational in vitro diagnostic TMA NAAT for detection of M genitalium.23 In all, 3,393 subjects (1,789 female and 1,604 male) were enrolled from 21 U.S. sites, including clinical research centers, emergency medicine, family planning, public health, STI and family medicine/obstetrics-gynecologic facilities. The study population included sexually active male or female subjects 14-years-old or older, with or without STI symptoms. Subjects with antibiotic treatment within 21 days of enrollment were excluded.
There were 3,300 subjects evaluable for infected status, and 11,556 specimens were collected and analyzed. Among 1,737 female subjects, four different specimens were collected (clinician-collected vaginal: n=1,709; patient-collected vaginal: n=1,724; endocervical: n=1,714; female urine: n=1,733). Among 1,563 male subjects, three different specimens were collected (urethral: n=1,563; penile meatal: n=1,554; male urine: n=1,559).
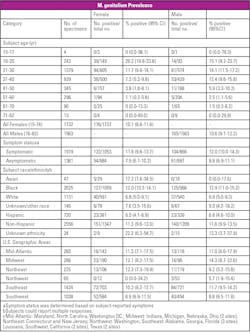
Although the subject age in the AMES Study spanned 15–82 in age, most subjects were between 18-40 (females: 84 percent, males: 70 percent).23 For either sex, approximately 61 percent of subjects were African American, approximately 77 percent were non-Hispanic, and approximately 74 percent were from southeast or southwest clinics.
For investigational assay sensitivity and specificity determinations, results from the investigational assay were compared with those from three different TMA NAATs that target M genitalium rRNA. One test amplified a 16S rRNA (alternate [Alt] TMA-1), using a different 16S rRNA locus than the investigational assay, and the other two amplified M genitalium 23S rRNA, each at a different locus (Alt TMA-2 and -3).23 For each assay, amplification and detection of the targeted nucleic acid region was a positive indicator. Each specimen was tested with the investigational assay, and then samples were further tested with Alt TMA-1 and -2.
If results were discordant, missing or invalid between the comparator tests, the specimen was further tested with Alt TMA-3 as a tiebreaker assay. Operators performing the Alt TMA assays were blinded to the investigational assay results and all patient-identifying information. Composite results for each specimen were then compared with the investigational assay result.
Prevalence of Mycoplasma genitalium STI
Overall M genitalium prevalence in the AMES study was 10.2 percent and 10.6 percent in female and male subjects, respectively.23 These results were consistent with recent reports of M genitalium prevalence estimates based on this TMA NAAT6,21,25 and were higher than some historical estimates.
Most subjects in the study (female, 61 percent; male, 55 percent) were symptomatic for STIs.23 M genitalium prevalence rates were comparable between symptomatic and asymptomatic subjects for both sexes (Figure).
The highest prevalence of M genitalium was observed in the 18-20-year-old category, in which more than a quarter of these younger female subjects were found to test positive for M genitalium infection compared with 15 percent for younger male subjects (Figure).23 In all other age categories, M genitalium infection prevalence rates were lower than in the 18–20-year cohort, were higher in male vs female subjects and declined with the age of subjects.
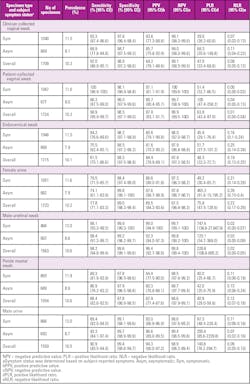
Overall M genitalium prevalence rates across all seven specimen types were highly consistent, ranging from 10.1 percent to 10.6 percent (Table).23 M genitalium prevalence was also higher in symptomatic vs asymptomatic subjects across all specimen types. In female subjects, prevalence was lowest (7.9 percent) in endocervical and urine samples from asymptomatic subjects, and highest (11.6 percent) in urine samples and patient-collected vaginal specimens from symptomatic subjects.
In male subjects, the lowest prevalence was found in urine specimens (8.7 percent) from asymptomatic subjects, and highest (12 percent) in urethral swab, self-collected, penile-meatal swabs and urine specimens from symptomatic subjects. Most subjects had positive investigational assay results for approximately two specimen types, and 56 percent of female and 68 percent of male subjects reported positive investigational assay results in all specimen types assessed, indicating the consistency of the assay across specimen types within individuals.
Assay performance
The investigational test exhibited a sensitivity for detection of M genitalium-infected subjects that ranged from 77.8 percent (female urine) to 98.9 percent (patient-collected vaginal swab) across the 7 specimen types (Table).23 These results suggest M genitalium infections are more likely to be missed in female subject urine samples compared with other specimen types. Overall specificity was greater than or equal to 97.8 percent for all specimen types. Sensitivity and specificity estimates were comparable in asymptomatic and symptomatic subjects for each specimen type.
Overall positive predictive values ranged from 82.9 percent to 96.4 percent and negative predictive values from 97.5 percent to 99.9 percent across all specimens, indicating high predictive value for the investigational M genitalium diagnostic assay (Table). Assay performance was similar among races and ethnicities of subjects for all specimen types.
Summary and conclusions
The AMES Study TMA NAAT investigational assay has been cleared by the FDA as a test for detection of M genitalium, which is an important advance in STI diagnostic technology. Lack of an FDA-cleared assay has hindered accurate M genitalium diagnosis and appropriate treatment, which has impaired patient outcomes and possibly fostered antibiotic resistance. Inadequate identification of M genitalium has delayed our understanding of the natural history of M genitalium infections and the long-term consequences of persistent infections.
The results of the AMES Study demonstrated that the assay can reliably assist physicians in identifying the M genitalium organism to be able to study its significance in further research studies. More studies are required to assess its significance in asymptomatic patients. The requirement for antibiotic resistance assays will help minimize the risk for development of antibiotic resistance.21
The FDA-cleared M genitalium test is a highly sensitive RNA based assay that can be performed in clinical settings with a short turnaround time for reporting results. This FDA-cleared testing method allows easy detection of M genitalium in a variety of clinical specimens from patients. The AMES Study demonstrated that assay sensitivity is robust for all specimens and that vaginal specimens had the highest detection sensitivity.
The study results confirm M genitalium prevalence rates are significant in sexually active young people, particularly in females, even in asymptomatic subjects. Both symptomatic and asymptomatic patients can be diagnosed with this FDA-cleared test. Questions remain about the significance of asymptomatic infections. Future research studies of asymptomatic subjects may be able to help determine the significance of M genitalium in such people, possibly guide treatment decisions and clarify the potential negative outcomes associated with asymptomatic M genitalium infections.
References
- Gaydos CA. Mycoplasma genitalium: accurate diagnosis is necessary for adequate treatment. J Infect Dis. 2017;216(suppl_2):S406-11.
- Andersen B, Sokolowski I, Østergaard L, et al. Mycoplasma genitalium: prevalence and behavioral risk factors in the general population. Sex Transm Infect. 2007;83:237-41.
- Oakeshott P, Aghaizu A, Hay P, Reid F, Kerry S, Atherton H, et al. Is Mycoplasma genitalium in women the “new chlamydia?” A community-based prospective cohort study. Clin Infect Dis. 2010;51:1160-6.
- Sonnenberg P, Ison CA, Clifton S, et al. Epidemiology of Mycoplasma genitalium in British men and women aged 16–44 years: evidence from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Int J Epidemiol. 2015;44:1982-94.
- Walker J, Fairley CK, Bradshaw CS, et al. The difference in determinants of Chlamydia trachomatis and Mycoplasma genitalium in a sample of young Australian women. BMC Infect Dis. 2011;11:35.
- Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-83.
- Gnanadurai R, Fifer H. Mycoplasma genitalium: a review. Microbiology. 2020;166:21-9.
- Centers for Disease Control and Prevention. 2015 Sexually Transmitted Diseases Treatment Guidelines. https://www.cdc.gov/std/tg2015/emerging.htm#myco. Last reviewed June 4, 2015. Accessed January 1, 2020.
- Manhart LE, Holmes KK, Hughes JP, Houston LS, Totten PA. Mycoplasma genitalium among young adults in the United States: an emerging sexually transmitted infection. Am J Public Health. 2007;97:1118-25.
- Seña AC, Lee JY, Schwebke J, et al. A silent epidemic: the prevalence, incidence and persistence of Mycoplasma genitalium among young, asymptomatic high-risk women in the United States. Clin Infect Dis. 2018;18:73-9.
- Wong C. Mycoplasma genitalium: challenges in diagnosis and treatment. Infectious Disease Advisor. https://www.infectiousdiseaseadvisor.com/home/topics/sexually-transmitted-diseases/mycoplasma-genitalium-challenges-in-diagnosis-and-treatment Published August 21, 2017. Accessed January 1, 2020.
- Hlatshwayo M, Reno HEL, Yarbrough ML. STI update: testing, treatment and emerging threats. Cleve Clin J Med. 2019;86:733-40.
- Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011:24:498-514.
- Conway R, Cook S, Soni S. Antibiotic treatment of Mycoplasma genitalium infection. The Pharmaceutical Journal. 2019;303:7928. https://www.pharmaceutical-journal.com/cpd-and-learning/learning-article/antibiotic-treatment-of-mycoplasma-genitalium-infection/20206592.article Published August 2019. Accessed January 1, 2020.
- Haggerty CL, Taylor BD. Mycoplasma genitalium: an emerging cause of pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2011;2011:959816 [Epub 2011 Dec 25].
- Trent M, Perin J, Gaydos CA, et al. Efficacy of a technology-enhanced community health nursing intervention versus standard of care for adolescent and young adult women with pelvic inflammatory disease. JAMA Netw Open. 2019;2:e198652.
- Wiesenfeld HC, Hillier SL, Meyn L, et al. Mycoplasma Genitalium – Is It a Pathogen in Acute Pelvic Inflammatory Disease (PID [abstract 004.6] Sex Transm Infect. 2013;89(Suppl 1):A34.
- Munoz JL, Goje OJ. Mycoplasma genitalium: an emerging sexually transmitted infection. Scientifica (Cairo). 2016;2016:7537318 [Epub 2016 Feb 29].
- Vallely LM, Egli-Gany D, Pomat W, et al. Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae, Mycoplasma genitalium, M. hominis, Ureaplasma urealyticum and U. parvum: a systematic review and meta-analysis protocol. BMJ Open. 2018;8:e024175.
- Ona S, Molina RL, Diouf K. Mycoplasma genitalium: an overlooked sexually transmitted pathogen in women? Infect Dis Obstet Gynecol. 2016;2016:4513089 [Epub 2016 Apr 24].
- Unemo M, Salado-Rasmussen K, Hansen M, et al. Clinical and analytical evaluation of the new Aptima Mycoplasma genitalium assay, with data on M. genitalium prevalence and antimicrobial resistance in M. genitalium in Denmark, Norway and Sweden in 2016. Clin Microbiol Infect. 2018;24:533-9.
- Kirkconnell B, Weinbaum B, Santos K, et al. Design and validation of transcription-mediated-amplification nucleic acid amplification tests for Mycoplasma genitalium. J Clin Microbiol. 2019;57:e00264-19.
- Gaydos CA, Manhart LE, Taylor SN, et al. Molecular testing for Mycoplasma genitalium in the United States: results from the AMES Prospective Multicenter Clinical Study. J Clin Microbiol. 2019;23;57:e01125-19.
- Roche receives FDA clearance to expand testing menu on cobas 6800/8800 Systems for sexually transmitted diseases [news]. Pleasanton, CA: Roche Molecular Diagnostics, Inc.; 23 May 2019. https://diagnostics.roche.com/us/en/news-listing/2019/roche-receives-fda-clearance-to-expand-testing-menu-on-cobas-6800-8800-systems-for-sexually-transmitted-diseases.html Accessed January 1, 2020.
- Le Roy C, Pereyre S, Hénin N, Bébéar C. French prospective clinical evaluation of the Aptima Mycoplasma genitalium CE-IVD assay and macrolide resistance detection using three distinct assays. J Clin Microbiol. 2017;55:3194-200.
About the Author

Charlotte Gaydos, MS, MPH, DrPH, F(AAM)
is a Professor in the Department of Medicine, Infectious Disease Division, Johns Hopkins University School of Medicine, as well as President of the International Union Against Sexually Transmitted Infections (IUSTI), and member of the Center for Global Health. She received her M.S. in medical microbiology from West Virginia University, and her MPH and DrPH in immunology and infectious diseases were from Johns Hopkins University School of Public Health. She is the Director of the University International STI, and Respiratory Diseases, Research Laboratory.