Clinical and diagnostic considerations for diabetes mellitus
Earning CEUs
For a printable version of the January CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
JANUARY LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Recall the signs, symptoms and risk factors of the development of diabetes mellitus.
2. Describe the pathophysiology of the classifications of diabetes mellitus.
3. Discuss lab values in the diagnosis of diabetes mellitus.
4. Discuss the HgA1C test, its limitations and factors in interpreting results.
The term diabetes, derived from the Greek word diabainein, meaning “to pass through,” refers to any condition which is associated with the production of large amounts of urine. When the polyuria is associated with hyperglycemia, the term Diabetes Mellitus (DM) is used (Mellitus, from the Latin meaning “sweetened with honey”). The hyperglycemia results from reductions in insulin production, secretion or action on many cell types and with long-term hyperglycemia, there can be damage to nerves, blood vessels, retinas and kidneys.
It is estimated that the direct cost of diagnosis and treatment of Diabetes Mellitus in the United States is over $350 billion annually. The cost of undiagnosed diabetes and the long-term sequelae is many times that number. Therefore, efficient approaches to the diagnosis of this heterogeneous group of diseases are crucial to identify and treat patients early in their disease cycle to reduce the high cost of managing the late-stage complications and to improve patient outcomes. There are multiple types of Diabetes Mellitus, with classification being based on the mechanisms causing hyperglycemia. As defined by the American Diabetes Association Standards of Care, the following categories are recognized:
Type 1 diabetes: due to autoimmune b-cell destruction, usually leading to absolute insulin deficiency. Autoimmune markers include islet cell autoantibodies and autoantibodies to GAD (GAD65), insulin, the tyrosine phosphatases IA-2 and IA-2β, and ZnT8. Type 1 diabetes is defined by the presence of one or more of these autoimmune markers. The disease has strong HLA associations, with linkage to the DQA and DQB genes.
Type 2 diabetes: due to a progressive loss of β-cell insulin secretion frequently on the background of insulin resistance.
Gestational diabetes mellitus (GDM): diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes prior to gestation.
Specific types of diabetes due to other causes: e.g., monogenic diabetes syndromes (such as neonatal diabetes and maturity-onset diabetes of the young [MODY], diseases of the exocrine pancreas (such as cystic fibrosis and pancreatitis), and drug- or chemical-induced diabetes (such as with glucocorticoid use, in the treatment of HIV/AIDS, or after organ transplantation).1
How is DM diagnosed?
Patients in all categories of DM manifest symptoms of increased plasma glucose, resulting in polydipsia, polyuria, polyphagia with weight loss, increased numbers of yeast infections and impairment of growth. Uncontrolled hyperglycemia often leads to ketoacidosis or lactic acidosis from nonketotic hyperosmolar syndrome.
The underlying common element is a lack of insulin response at the end-organ cell receptors. This may result from a decreased production of insulin due to autoimmune destruction of the beta cells of the pancreas. Other patients with DM have a resistance to the action of insulin, sometimes associated with metabolic syndrome and obesity. The basis of the abnormalities in carbohydrate, fat, and protein metabolism results from inadequate insulin secretion and/or diminished tissue responses to insulin at one or more points in the complex pathways of hormone action. Impairment of insulin secretion and defects in insulin action frequently coexist in the same patient, and it is often unclear which abnormality, if either alone, is the primary cause of the hyperglycemia.2
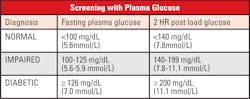
If a patient presents in a hyperglycemic crisis, or with clear signs and symptoms of chronic hyperglycemia, the diagnosis of DM can be confirmed by a single random plasma glucose which exceeds 199 mg/dL. Without this severe clinical presentation, the diagnosis depends on two abnormal glucose values.7
Criteria for the diagnosis of diabetes (at least one of the following criteria are met):
- After at least 8 hours of no caloric intake, the plasma glucose is ≥126 mg/dl
- After the administration of a 75-gram glucose oral challenge, the plasma glucose after two hours is ≥200 mg/dl
- A1C ≥6.5 percent employing a method that is NGSP certified and standardized to the DCCT assay
- In the clinical setting of marked hyperglycemia, a random plasma glucose ≥200 mg/dL alone is sufficient.
1. Testing should be considered in overweight or obese (BMI ≥25 kg/m2 or ≥23 kg/m2 in Asian Americans) adults who have one or more of the following risk factors:
• Women with polycystic ovary syndrome
• Physical inactivity
• Other clinical conditions associated with insulin resistance (e.g., severe obesity, acanthosis nigricans)
2. Patients with prediabetes (A1C ≥5.7 percent) should be tested yearly.
3. Women who were diagnosed with GDM should have lifelong testing at least every 3 years.
4. For all other patients, testing should begin at age 45 years.
5. If results are normal, testing should be repeated at a minimum of 3-year intervals, with consideration of more frequent testing depending on initial results and risk status.1
What is prediabetes?
There are a group of patients that do not fall into the “Normal” or the “Diabetic” categories when tested for fasting glucose or a 2-hour post load glucose challenge. These patients are recognized as having a relatively high risk of developing DM and cardiovascular disease in the future. They frequently are obese, with increased lipids, particularly triglycerides, and hypertension. Patients with prediabetes often have near-normal glycated hemoglobin levels and can only be classified using the standardized OGTT.
What is the role of HbA1c in the diagnosis and management of DM?
Two laboratory assays play a role in the diagnosis and monitoring of diabetic patients: plasma glucose and HbA1c. With glucose, results from many different assay methods can be combined to create a longitudinal patient record, with widely accepted reference ranges and standardized assays. In contrast, patient results for HbA1c should not be combined from different methodologies, due to variations in interference from variant hemoglobins. Red blood cell (RBC) survival times should also be considered, with shortened survival resulting in artificial lowering of the HbA1c.
The HbA1c result is used to provide an estimation of the patient’s glycemic control over the last two to three months, assuming the RBC’s have an average circulating lifespan of 120 days. During that time period, glucose in the blood permanently binds to the hemoglobin in the RBC by the Amadori rearrangement forming HbA1c from the wild type (or typical) HbA. The higher the level of circulating glucose the higher the percentage of HbA1c will be formed, in turn, an average estimated glucose level (eAG) can be calculated from the percentage of HbA1c.
The American Diabetes Association (ADA) has published Standards of Care for HbA1c:
1. To avoid misdiagnosis or missed diagnosis, the A1C test should be performed using a method that is certified by the NGSP and standardized to the Diabetes Control and Complications Trial (DCCT) assay.
2. Marked discordance between measured A1C and plasma glucose levels should raise the possibility of A1C assay interference due to hemoglobin variants (i.e., hemoglobinopathies) and consideration of using an assay without interference or plasma blood glucose criteria to diagnose diabetes.
3. In conditions associated with an altered relationship between A1C and glycemia, such as sickle cell disease, pregnancy (second and third trimesters and the postpartum period), glucose-6-phosphate dehydrogenase deficiency, HIV, hemodialysis, recent blood loss or transfusion, or erythropoietin therapy, only plasma blood glucose criteria should be used to diagnose diabetes.1
If one uses HbA1c with a diagnostic cutoff of 6.5 percent, the diagnostic sensitivity for DM is 30 percent, meaning, if only HbA1c is ordered, there is a 70 percent chance of patients being missed in the diagnosis of DM.3
Interpreting HbA1c results
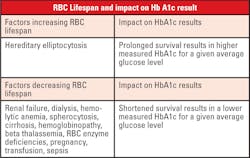
When interpreting HbA1c results, one must consider biologic variation of this marker. There is normal genetic variation in the rate of hemoglobin glycation. Conditions that prolong or shorten RBC survival will also disrupt the direct relationship of average glucose to the level of HbA1c. Patient age must also be considered, as levels increase with aging. Lastly, patient ethnicity plays a role, as levels are higher in African Americans at the same degree of glycemic control.
The choice of methodology of an assay for HbA1c should take into consideration the following interferences:
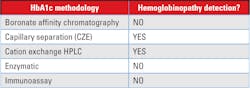
1. Analytical interference: Most newer methods for HbA1c have minimal analytical interference from the presence of the major hemoglobin variants (HbS, HbC, HbE, HbD) in the specimen. The reader is referred to the NGSP website for a more detailed table by manufacturer and methodology.
2. Clinical interference: There are clinical conditions that will limit the ability to use the HbA1c value as an estimate of the degree of glycemic control. “This issue is of particular concern when using assays for HbA1c (e.g. immunoassay) that will produce an HbA1c result for homozygous Hb variants, without providing information that a Hb variant is present in the sample.”4
The current interpretation of HbA1c values, which corresponds to the calculated (eAG), assumes that the RBC life span is the same for all patients. However, even modest variation in red cell survival, which would not be apparent in routine hematological studies, could have a significant impact on the HbA1c level.1 Therefore, the detection of some of the more common causes of decreased (or increased) RBC survival would be important in determining whether the HbA1c level was an accurate reflection of a patient’s level of glycemic control. In general, a shorter RBC life span would yield lower levels of HbA1c at a given average whole blood glucose concentration as compared to that of a normal patient.
Extrinsic causes of decreased RBC survival include pernicious anemia, acquired hemolytic anemia, pregnancy, nephritis, hepatic disease, burns, sepsis, and anemia associated with malignancy. Intrinsic causes include hemoglobinopathy, paroxysmal nocturnal hemoglobinuria, congenital hemolytic jaundice and elliptocytosis. Renal and hepatic disease may be detected by scrutiny of the results of routine serum chemistry profiles. Hemolytic anemia is rare and may be suspected with a normocytic, normochromic pattern of anemia. Rarely will a patient with diabetes have testing which is specifically focused on determining if red cell survival is diminished due to congenital causes, with the most common condition being the presence of a hemoglobinopathy.6
Most methods are free from analytical interference from common hemoglobinopathies; however, the clinical interference may not be known if the patients’ result does not indicate the presence of a hemoglobinopathy or other disease state that can alter the RBC lifespan.5
Estimating glycemic control from HbA1c alone is applying a population average to an individual, which can be misleading. Although the mean of the average glucose concentration (AGC) is correlated with the HbA1c, there is a significant degree of inter-individual variation in AGC at the medical decision point of HbA1c (6.5 percent) which includes some AGC values well within the non-diabetic range. Likewise, some patients having HbA1c levels below 6.0 percent have AGC values which are associated with poor glycemic control. Therefore, should a single HbA1c less than 5.7 percent be relied on to rule out pre-diabetes or diabetes mellitus? If the patient has an unsuspected condition that results in shortened RBC survival, this will falsely lower the HbA1c, to an extent that the patient will appear to be euglycemic. It is advisable that the method chosen for screening give information related to the presence of hemoglobin abnormalities, and that with homozygous or double heterozygous conditions, an alternate test be chosen for screening and monitoring of therapy. HbA1c can no longer be the only quality monitoring element for all diabetics. The key to effective utilization of HbA1c is knowing when this marker is most likely to be an inaccurate indirect indicator of glycemic control due to reductions in red cell circulation times. The choice of an analytical method for HbA1c is important, as methods that do not identify the presence of abnormal hemoglobin molecules may give erroneous results that, when reported, can wrongly indicate better glycemic control that truly exists for that patient.
Summary
Diabetes mellitus has several main subtypes, all having periods of hyperglycemia, and all, if left untreated, will result in damage to the kidneys, optic nerves, peripheral nerves and blood vessels. A third of patients with Type 1 DM have an initial presentation with ketoacidosis or lactic acidosis, while Type 2 and Gestational DM are more commonly found to have hyperglycemia on routine screening. The ADA has published criteria for the diagnosis of DM. Using these criteria, some patients do not fall into the “Diabetic” category, but who have Impaired Fasting Glucose or Impaired Glucose Tolerance. These prediabetic patients are usually obese, and have hyperlipidemia, low HDL and hypertension. Many of these patients will see reductions in fasting and post load glucose values with a proper weight loss regimen and regular exercise schedule. The category of Gestational Diabetes Mellitus has a specific screening algorithm, and these patients require monitoring of plasma glucose levels at three-year intervals during their lifetime.
Guidelines for screening and monitoring of Type 1 and Type 2 diabetics now includes the measurement of HbA1c levels. While there are many techniques utilized by labs for this analyte, there is an increasing awareness of the role played by RBC survival times in the creation of the glycated hemoglobin molecules. While some of the causes of altered RBC survival are readily apparent in the patient’s clinical presentation, the presence of hemoglobinopathies or thalassemia may go unrecognized. In patients with homozygous or doubly-heterozygotic hemoglobinopathies, the red cell survival may be reduced to a degree that precludes the use of HbA1c as a marker of hyperglycemia. The ADA has issued guidelines that, “In conditions associated with an altered relationship between A1C and glycemia, such as sickle cell disease, pregnancy (second and third trimesters and the postpartum period), glucose-6-phosphate dehydrogenase deficiency, HIV, hemodialysis, recent blood loss or transfusion or erythropoietin therapy, only plasma blood glucose criteria should be used to diagnose diabetes.”1 It is therefore critical that patient testing for HbA1c should be performed initially using a method which can detect the presence of abnormal hemoglobin molecules or thalassemia’s, and that the presence of these abnormalities is communicated to the ordering physicians to determine the effect, if any, on the measured HbA1c. The degree of glycemic control should then be measured using glycated albumin or continuous glucose monitoring meters.
REFERENCES
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes 2019. Diabetes Care 2019;42(Suppl. 1): S13–S28.
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2004 Jan; 27(suppl 1): s5-s10.
- Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988– 2006. Diabetes Care 2010; 33:562–568
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-393
- Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem. 2001; 42:153-163.
- Lohmann, T. The impact of red blood cell lifespan on HbA1c measurement. Medical Laboratory Observer, June 24, 2019.
- Selvin E, Wang D, Matsushita K, Grams ME, Coresh J. Prognostic implications of single-sample confirmatory testing for undiagnosed diabetes: a prospective cohort study. Ann Intern Med 2018; 169:156–164
- Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The Fallacy of Average: How Using HbA1c Alone to Assess Glycemic Control Can Be Misleading. Diabetes Care. 2017;40(8):994–999. doi:10.2337/dc17-0636
About the Author

Thomas Lohmann, MD
Serves as Director of Medical and Scientific Affairs for Sebia-USA. He is board certified in Anatomic and Clinical Pathology, and has 40 years of experience in Laboratory Medicine.