Understanding the CDC’s updated HIV test protocol
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Describe the history of HIV testing and the purpose for developing an HIV algorithm.
2. Describe the limitations of the first testing methods that were produced for the detection of HIV.
3. Discuss the need for an updated algorithm.
4. Identify the new objectives in the current algorithm and describe the test methods involved.
After the human immunodeficiency virus type 1 (HIV-1) was identified as the cause of acquired immunodeficiency syndrome (AIDS) in the early 1980s, publicly and privately funded scientists worked quickly to develop tests that could detect the antibody to the retrovirus. Although imperfect, these tests have been used for the clinical diagnosis of HIV infection in both symptomatic and asymptomatic patients and for blood-donor screening for three decades.1
In 1985, the Food and Drug Administration (FDA) approved for marketing the first antibody-based human immunodeficiency virus (HIV) screening test, an HIV-1 enzyme immunoassay (EIA). In the same year, the Centers for Disease Control and Prevention (CDC) released an interim HIV test algorithm and guidelines, and four years later published its first formal guidelines. The algorithm is a recommended step-by-step testing process that is designed to overcome the limitations of any one test. It is also a way of combining the best attributes of several tests to get more accurate results than any single test can deliver. Under the algorithm, which was updated slightly in 1992, if an initial antibody assay was repeatedly reactive it was to be followed by a supplemental test. For 23 years, the protocol required labs to perform either a confirmatory Western blot (WB) or immunofluorescent antibody (IFA) assay on repeatedly reactive specimens.2 Market dynamics made WB the favorite.
But in June 2014, the CDC changed its algorithm to one that no longer recommended the use of the WB to confirm the presence of HIV-1 antibodies (though the test is still used for a few other applications). Technological advancements had led to the approval of an HIV-1/HIV-2 antibody differentiation test that was more sensitive than the WB, especially early in an infection. Moreover, it was a rapid test, which shortened the turnaround time for confirmation of HIV infection. Thus, technological advancements in testing championed by the private sector made the CDC’s original blood-specific HIV
algorithm obsolete.3,4
Generations of HIV tests
To detect HIV, a lab either must detect markers of the human immune cellular response to the infection, usually antibodies, or the genetic material of the virus itself, using a nucleic acid amplification test (NAT), or a derivation such as a polymerase chain reaction test (PCR). From 1985 to 1999, the year the first molecular tests obtained FDA approval, antibody tests and some antigen tests basically comprised the HIV test arsenal.
EIAs became the most widely used antibody tests in the United States due to their high sensitivity and standard methodology, making them suitable for high-volume testing. EIAs are designed to detect antibodies (immune cells) or antigens (proteins on the virus that stimulate an immune response) that indicate the presence of HIV infectivity by producing a color change caused by a reaction to an enzyme. The FDA has licensed approximately 10 EIAs.
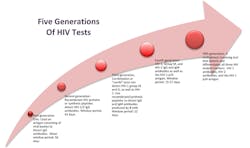
Since 1985, when commercial immunoassays for HIV-1 detection first became available, there have been five new generations of tests for screening and diagnosis (Figure 1). (The CDC has published a guide that describes the qualities and differences among the first four generations of tests.5) Each new generation of HIV assays further reduced the detection window period—the time between potential exposure and an accurate test result—and, therefore, the time to the diagnosis and treatment of early infections.
- First-generation EIAs used an antigen consisting of viral lysates to detect immunoglobulin G (IgG) antibodies. The window period of infectivity detection was 56 days.
- Second-generation tests relied on recombinant HIV proteins or synthetic peptides to detect HIV-1/2 IgG antibodies. The window period was reduced to 42 days.
- Third-generation tests are basically combination or “combi” tests that can detect HIV-1 Group M (for “major,” the common U.S. AIDS-causing strain) and O (for “outlier,” the rare African strain), as well as HIV-2. They also use recombinant/synthetic peptides to detect IgG antibodies, as well as immunoglobulin M (IgM) antibodies produced by B cells. The window period was reduced to 22 days.
- Fourth-generation assays, introduced in 2000, could detect HIV-1, Group M, and HIV-2 IgG and IgM antibodies, as well
as the HIV-1 p24 antigen. This advancement enabled labs to detect infection in 15 to
17 days. - The first fifth-generation assay is a multiplexed screening test that detects and differentiates all three HIV analyte markers: HIV-1 antibodies, HIV-2 antibodies, and the HIV-1 p24 antigen.
While the Western blot was for two decades the confirmatory test of choice in the majority of U.S. laboratories, that same time period saw HIV test manufacturers pouring millions of dollars into research to improve first-line tests. They found new methods to make antibody-based EIAs more sensitive and specific and began to develop different technologies to detect both antibodies and antigens, to differentiate infection by type, and to shorten the time to results.
WB: popular but problematic
The first WB kit for HIV-1 was licensed in the U.S. in 1991, and as a result of its apparent reliability and adequate specificity, WB assays proliferated and outpaced IFAs in popularity. The test, made from the inactivated virus itself, was named, in a sort of play on words, after the Southern blot, a technique used for DNA detection developed by Edwin Southern. In the WB technique, a mixture of proteins is subjected to gel electrophoresis, which uses electricity to separate DNA, RNA, or proteins based on molecular weight/type as they migrate through a gel matrix. These results are then transferred to a membrane producing a band for each protein. The membrane is then incubated with antibodies specific to the protein of interest.6
By 1999, eight years after the first WB test was approved, there were five major WB products on the market. But despite its popularity and critical position in the protocol, the test had it vulnerabilities. For starters, it was not easy to manufacture and involved significant training and a labor-intensive process to perform. It also took two to four days to produce results—visible protein bands that did not always lend themselves to easy interpretation, especially if there was any cross-contamination, background noise, or an impure gel matrix. Finally, the WB was vulnerable to missing early cases.
One large study of 3.6 million tests comparing the WB with IFA found that the use of the IFA had 13 times fewer indeterminate samples than the WB. And when pregnant women’s HIV tests were repeatedly reactive on an EIA, they were more likely to be negative or indeterminate on the WB.7 What’s more, the WB sometimes confused the relatively rare HIV-2 type for the common HIV-1 strain. The CDC reported in 2011 that 60 percent of a study group of 163 HIV-2 cases were HIV-1 reactive on a WB.
As new generations of first-line assays came on the market, laboratory supervisors, including those at public health laboratories (PHLs) in states with known hot spots for HIV incidence, began to notice a disturbing trend. More specimens were producing repeatedly reactive results on initial screening but negative or indeterminate results on the WB. A significant number of those specimens came from people known to be at high risk.
PHLs experiencing unconfirmed HIV diagnostic results with the old algorithm often became aware of clinical manifestations in the person being tested that caused them to suspect that the patient could be in an acute, or early, stage of HIV infection. If a laboratory issued a negative or indeterminate report, it would be up to the public health provider to advise the person that he should return to provide another blood sample in two or three weeks to capture seroconversion. (The variability of individual immune responses also played a part, considering that the body starts producing antibodies between two and 12 weeks of infection). But many people who might have been infected with HIV did not return after the initial visit, or the hospital lost touch with them entirely.8
Revisiting the algorithm: the momentum gathers
The problem of the WB missing early acute-stage HIV infections, as well as missing HIV-2 cases—and hospitals losing touch with patients—would eventually lead the CDC in 2008 to launch a six-year project to change its recommended HIV test algorithm. Suggestions that the CDC consider changing the protocol actually came three years earlier, in 2005, when the CDC and the Association of Public Health Laboratories (APHL) co-hosted the first HIV Diagnostics Conference. Some 200 researchers, laboratorians, and industry representatives attended the meeting, which was billed as a forum for sharing “the latest information on testing technologies and alternative methods to increase the uptake of testing and diagnosis of persons with HIV infection,” according to a summary of the 2005 conference.9
Several presentations dealt with the vulnerabilities of the WB compared with other assays, and others suggested different tests or alternative algorithms. S. Michele Owen, PhD, head of the Lab Branch of the Division of HIV/AIDS Prevention of the CDC, reported the results of a study of 713 specimens that were tested by an initial EIA, retested on an alternate EIA if the original test was non-reactive, and then tested again using WB if the second test was reactive. Results showed that 675 specimens were initially reactive and deemed HIV-1positive. Of the 38 non-reactive specimens, 26 were reactive on the alternate EIA, but of that number, 17 were reactive on the WB, zero were non-reactive, and nine, or nearly one-third, of the repeatedly reactive specimens were indeterminate.
An official with the American Red Cross (ARC) reported that of 12.4 million blood donations from 1989 through 1999, 11,080 were EIA repeatedly reactive, and of these, 7.1 percent were WB positive, 46.6 percent were WB indeterminate, and 46.3 percent were WB negative. She explained that the rate of indeterminate and negative WB results could be partly attributed to an FDA requirement that any background discoloration or band must be reported in addition to clearly viral bands.
Several presentations explored other algorithm options that would reduce or eliminate the use of WB. These included using NAT or more advanced-generation EIA for supplemental confirmatory testing; or using combinations of rapid tests; or using a particular rapid test in combination with HIV-1 and HIV-2 assays and the WB. One presentation concluded that NAT testing could not completely replace the WB, given that some HIV-infected people have non-detectable viral loads. A CDC study found that up to 3.3 percent of serology-positive test subjects were NAT negative.
Overhauling the algorithm: the critical mass
The process of overhauling the algorithm began in earnest in 2008-2009, with the publication of an APHL status report that was based on the findings of a group that analyzed various algorithms that labs were using and identified the pros and cons of different schemes.
At the 2010 HIV Diagnostics Conference in Atlanta, Bernard Branson, MD, then the Associate Director for Laboratory Diagnostics in the CDC’s Division of HIV/AIDS Prevention, announced that the organization was seriously considering a “multispot test” that produced reactive spots on a cube that enabled a lab to distinguish HIV-1 from HIV-2.
At the Diagnostic Conference of 2012, a group of public and private scientists presented draft recommendations and a new algorithm for diagnostic HIV testing. The recommendations were designed to diagnose people earlier in infection; to better and more accurately distinguish HIV-1 from HIV-2; and to get results back to people sooner. One study showed that people with early infections, particularly those who are antigen-positive but not yet antibody-positive, were found to have a higher concentration of the virus in their bodies and thus were more likely to infect others.10 There was also mounting evidence that the sooner an individual started therapy, the less damage was wrought on his or her immune system, and thus, the greater likelihood of an increased life span.
The objective to more accurately diagnose HIV-2 was inspired by the fact that if a patient is misdiagnosed as being infected with HIV-1, he or she might be treated with ineffective drug therapy. Some of the reverse transcriptase inhibitors used to treat HIV-1 are effective in fighting HIV-2. However, other classes of drugs, such as the protease inhibitors, are not.
And getting results back on the same day instead of a week later is important because a significant number of people who come in for screening leave their specimen but fail to return to obtain results. At the 2010 conference, Dr. Branson said that at the time about 20 percent of those tested in the U.S. never obtained WB confirmatory results.
Revised recommendations
After years of development, the CDC in June 2014 published a new HIV test algorithm and recommendations. (Figure 2) The new protocol is intended to help laboratories detect chronic, or established, infections, as well as acute, or new, infections up to a month sooner than the previous testing protocol.11 Thus, public health officials and clinicians can concentrate on people who are in the early stage of HIV infection.12
The revised algorithm enables the detection of the p24 antigen as the viral load ramps up. By exposing infectivity earlier, the revised algorithm helps clinicians get HIV patients into treatment faster and supports public health efforts to restrict the spread of the disease by making HIV patients’ sexual partners aware that they may be at risk and should be tested. The protocol also differentiates HIV-1 from HIV-2 and eliminates most indeterminate results because of the greater sensitivity of today’s supplemental test technology.
The first level of testing is with an HIV-1/2 “combo” immunoassay, which can be either a fourth- or fifth-generation test. If the serum specimen is positive in the first-line test—meaning the qualitative detection of HIV-1 p24 antigen or HIV-1/2 antibodies—it is subject to the HIV-1/2 antibody differentiating assay.
A positive result of the differentiating assay for either HIV-1 or HIV-2 will be interpreted and diagnosed. A negative or indeterminate test will require a NAT test, which will confirm the accurate detection of an early infection or indicate a false positive by the fourth-generation test. A positive test will trigger a diagnosis of acute HIV-1 infection. A negative result will indicate the individual is HIV-negative.
The FDA has approved three non-differentiating, fourth-generation “combo” assays: (1) the Bio-Rad HIV-1/2 Ag/Ab EIA; (2) the Abbott Architect HIV Ag/Ab chemiluminescent assay; and (3) the ADVIA Centaur Ag/Ab CIA.
The FDA has approved one combo differentiating fifth-generation assay, the BioPlex 2200 HIV Ag-Ab. This assay detects and differentiates antibodies to HIV-1 and HIV-2, as well as the HIV-1 p24 antigen. Bio-Rad’s Multispot HIV-1/2 assay is scheduled to be withdrawn from the market as of December 2016.
The FDA has approved a number of “rapid tests,” including (a) the Bio-Rad Geenius HIV-1/2 Supplemental Assay; (b) the Alere Determine HIV-1/2 Ag/AB Combo assay; (c) the INSTI HIV-1/2; (d) the DPP HIV-1/2; and the (e) OraQuick Advance HIV-1/2.
Labs may continue to use a third-generation “combi” HIV-1/2 antibody-only assay, but they run the risk of missing cases in which the patient is HIV antibody-negative but HIV-1 antigen positive.13
It is clear that the pace of technological advancements in HIV testing is continuing to accelerate. To keep abreast of the changes, the CDC is expected to recommend more improvements to the HIV test algorithm in coming years.
References
- Starr D. Blood: An Epic History of Medicine and Commerce. 2nd edition, New York, NY: Perennial/HarperCollins Publishers. p. 300.
- Centers for Disease Control and Prevention. Interpretation and use of the Western blot assay for serodiagnosis of human immunodeficiency virus type 1 infections. MMWR. July 21, 1989 / 38(S-7);1-7. www.cdc.gov/mmwr/preview/mmwrhtml/00001431.htm (for the 1989 algorithm and related recommendations). Accessed December 17, 2015.
- Some of the information used in this article was derived from a previous article by the author published in this journal. Kapler R. HIV test algorithm matches protocol with latest technology. MLO. 2015;47(4):38-40.
- The original algorithm is still used for testing other bodily fluids.
- Centers for Disease Control and Prevention. Advantages and disadvantages of different types of FDA-approved HIV immunoassays used for screening by generation and platform. www.cdc.gov/hiv/pdf/testing_Advantages&Disadvantages.pdf. Accessed December 17, 2015.
- Mahmood T, Yang P-C. Western blot: technique, theory and trouble shooting. N Am J Med Sci. 2012;4(9): 429–434. www.ncbi.nlm.nih.gov/pmc/articles/PMC3456489. Accessed December 17, 2015.
- Summary of the 2010 HIV Diagnostics Conference. http://www.hivtestingconference.org/hivtesting2010/PDF/2010HIVDiagnosticsConfSummary.pdf. Accessed December 17, 2015.
- Kapler R. HIV test algorithm matches protocol with latest technology. MLO 2015;47(4):38-40.
- Summaries of the 2005, 2007, 2010 and 2012 HIV Diagnostic Conferences, which contain extensive data on the test algorithm development, can be found at www.hivtestingconference.org.
- Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. NEJM. 2011;365:493-505.
- The three stages of HIV infection are acute (or early) HIV infection, chronic (or established) HIV infection, and acquired immunodeficiency syndrome (AIDS).
- Bransom BM, Own SM, Wesolowski LG, et al. Laboratory testing for diagnosis of HIV infection: updated recommendations. www.cdc.gov/hiv/pdf/hivtestingalgorithmrecommendation-final.pdf. Accessed December 17, 2015.
- Much of this information was provided by Berry Bennett, MPH, head of the Retrovirology Unit of the Florida Bureau of Public Health Laboratories, during his lecture on 9/18/15 at the Bio-Rad CE Event, East Elmhurst, NY.