To take the test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Discuss the original focus of laboratory quality.
2. Describe the evolution of how quality monitoring is changing.
3. Differentiate between quality control and quality assurance.
4. List the steps of a complete quality management system (QMS).
The concept and definitions of quality in laboratory medicine have been steadily evolving throughout the second half of the twentieth century right up to the present day. Originally focused on the analytical phase of testing, quality was defined primarily by measurements of the accuracy, precision, and reproducibility of test results, determined by performing quality control (QC) on the testing process. Professional as well as governmental standards of acceptable performance were originally based on this paradigm.
However, in recent years, the concept of quality monitoring for laboratory testing has evolved to encompass the total testing process, also known as TTP. Beginning with test ordering and ending with result reporting, TTP encompasses the pre-analytical, analytical, and post-analytical phases of testing. Monitoring this entire process is the basis of quality assurance (QA).1 QA is thus defined as the overall monitoring program that ensures that the final test results reported by the laboratory are as correct and timely as possible; with QC being one component of a program that includes standards for specimen collection, instrument maintenance, environmental monitoring, personnel training, competency and safety, data management, and record keeping.
Today, the concept of quality laboratory service continues to evolve enabled by rapid advances in digital technology, new service delivery models, the decentralization of patient contact, and the ability to meet the demands of non-physician patient test ordering and reporting.
Quality management
Quality control is designed to detect and correct deficiencies in a laboratory's internal analytical process prior to the release of patient test results in order to ensure the quality of the work reported by the laboratory. Quality control is a measure of precision, or how well the measurement system reproduces the same result over time and under varying operating conditions. Laboratory quality control material is usually run at the beginning of each shift, after an instrument is serviced, when reagent lots are changed, after calibration, and whenever patient results seem inappropriate. The effectiveness of quality control is assessed on a per test basis.2
Quality assurance is a more broadly focused ongoing program for auditing all of an organization’s processes and systems; it involves setting quality goals, deciding whether or not these goals have been achieved, and implementing corrective action if these goals have not been reached. It includes auditing the effectiveness of policies and procedures and encompasses all phases of testing and general administration. There are no limits for quality assessment audits as these go well beyond the more test-specific focus of quality control.2
The CLIA regulations address specific quality assurance requirements. The Code of Federal Regulations (42 CFR 493) states that laboratories “must establish and follow written policies and procedures for a comprehensive quality assurance program that is designed to monitor and evaluate the ongoing and overall quality of the total testing process.” The QA program must accomplish the following:
- Assess the effectiveness of the lab’s policies and procedures
- Identify and correct problems
- Ensure the accurate, reliable, and prompt reporting of test results
Ensure the adequacy and competency of the staff
The changing landscape for quality management
While the management skills needed to lead a laboratory that provides quality patient care are well understood, the defining components of quality care itself are not static. Whether discussing the traditional issues of test accuracy, turnaround time, specimen acquisition, or result reporting, the operating environment around these activities is changing, including new testing and data technology and the way tests are performed and reported; new treatment protocols requiring new tests; physician needs for immediate digital access; legislation regarding laboratory fees and CLIA regulations; patient expectations; labor shortages; and changing demographics that will affect future demand for laboratory services. Additional changes include technological advances that have led to an increasingly visible and active role of the laboratory within healthcare delivery systems, the rapid decentralization of laboratory testing that has led to the rise of point-of-care testing (POCT) and retail medicine, as well as remote wearable technology allowing accurate mobile test monitoring. These developments place added responsibilities on the central laboratory to ensure that the quality of testing is maintained.
Thus, the requirements to maintain “quality patient services” even in remote settings that include pharmacies and department stores, as well as large non-medical employers such as Amazon, require a re-engineering of how reliable testing and reporting are maintained.
In Rothenberg’s “Adapting to change: How resilient is your laboratory?”, he notes, “These times call for more than good management, they call for good leadership, but leadership that is more adaptive and agile than ever before. These times call for leadership that understands change and can adapt by creating an organizational culture of quality through resilience, enabling their laboratory operation to not only survive but prosper and grow, maintaining quality service.”3
The quality management system
An organized quality management system (QMS), which oversees the entire laboratory operation, is very important for achieving optimal laboratory performance. The quality management system is not only concerned with monitoring QC/QA programs but should also include administrative considerations that may indirectly influence the quality and efficiency of the laboratory operation.4 A QMS is officially defined as “coordinated activities to direct and control an organization with regard to quality.” This definition is used by the International Organization for Standardization (ISO) and by the Clinical and Laboratory Standards Institute (CLSI).
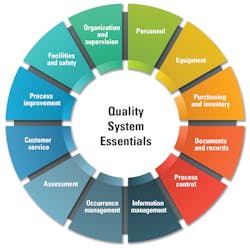
The quality management system model illustrated here organizes all of the laboratory activities into twelve Quality System Essentials (QSE), which are a set of coordinated activities that serve as building blocks for quality management. Each must be addressed if overall laboratory quality improvement is to be achieved.[i] The twelve QSEs are as follows: organization and supervision, personnel, equipment, purchasing and inventory, documents and records, process control, information management, occurrence management, assessment, customer service, process improvement, facilities and safety.5
1. Organization and supervision
The structure and management of the laboratory must be organized so that quality management policies can be established and implemented. The laboratory should prepare an organizational chart that includes all management and staff positions with functions and responsibilities delineated for each position. The current duties and responsibilities of the staff must be specified in written job descriptions including training required, competency assessments, and relevant experience. A quality manager should be designated to ensure the implementation and monitoring of the quality policies. Processes for monitoring will be needed to ensure that the system is working, and that benchmarks and standards are being met. This element is essential to the primary goal of a quality system, which is continuous improvement. The commitment and leadership by the laboratory director are crucial.
2. Personnel
Competent, trained, and motivated staff are the most important resource for a quality laboratory operation. To ensure this requirement is met, the laboratory must do the following:
- Hire an appropriate number of staff to cover the workload.
- Develop complete and thorough job descriptions for each employee.
- Train each employee in their specific duties and responsibilities.
- Provide orientation for new employees; this is separate from training. This is to ensure that new employees understand the culture of the laboratory; it is an investment in team building as well.
- Perform and record competency assessments on all personnel. It is management’s responsibility to verify that trained employees are sufficiently competent to do their work and maintain their competency over time. This can be done through direct observation of the personnel, records monitoring, and/or by analyzing the quality control or external quality assessment results.
- Provide opportunities for continuing education; new techniques or updates for existing methodologies will always occur.
- Conduct annual employee performance appraisals.
3. Equipment
Equipment management is one of the essential elements of a quality management system. Proper management of the equipment in the laboratory is necessary to ensure accurate, reliable, and timely testing. Choosing the right equipment, assuring that the staff is properly trained to use the equipment, and assuring that the new equipment works properly and receives proper maintenance are crucial. Equipment manuals should be available in the laboratory area for easy reference. An inventory of equipment including records of maintenance and repair should be maintained.
The benefits of a good equipment management program are many:
- Helps to maintain quality laboratory performance
- Improves the technologist’s confidence in the accuracy of testing results
- Lowers repair costs, as fewer repairs will be needed for a well-maintained instrument
- Lengthens instrument life
- Reduces the interruption of services due to breakdowns and failures
- Increases staff safety
- Produces greater customer satisfaction when service is not interrupted
4. Purchasing and inventory
Efficient and cost-effective laboratory operations require the uninterrupted availability of expected supplies and services. Proper management of purchasing and inventory can produce cost savings in addition to assuring accurate and timely reporting of laboratory results. The inability to test, even for a short time, is very disruptive to clinical care, impairing the quality of services offered.
Purchasing and inventory management procedures should be written and implemented to assure that all reagents and supplies are correctly selected, purchased, used, and stored in a manner that preserves integrity and reliability. The inventory should be kept up to date including information on receipt, storage, and issuance. Package inserts and safety data sheets (SDS) should be archived as part of recordkeeping.
5. Documents and records
Documents provide written information about policies, processes, and testing procedures and comprise the laboratory’s Policy and Procedure and Quality Improvement manuals. These manuals include all standard operating procedures (SOPs) that must be adapted to the laboratory’s role and testing menu. The SOPs, QC/QA procedures, specimen testing request forms, report forms, and other laboratory forms are all important components of these manuals, which document the quality management system. There should also be manuals related to laboratory safety. Examples of laboratory safety records include request forms, report forms, logbooks, quality control results, patient reports, critical communications, and notices from hospitals or public health authorities.
6. Process control
Quality control (QC) is an essential element of the quality management system. It monitors the processes related to the analytical phase of testing and detects errors in testing. These errors may be due to test system failure, adverse environmental conditions, or flawed operator performance. The goal of QC is to detect these errors and enable corrections before patient results are reported. The steps for implementing a QC program are as follows:
- Establish QC policies and procedures.
- Assign responsibility for monitoring and reviewing QC.
- Select appropriate QC material.
- Establish control ranges.
- Develop graphs to plot control values (Levey–Jennings charts).
- Establish a system for monitoring control values.
- Take immediate corrective action if needed.
- Maintain records of QC results and any corrective actions taken.
7. Information management
Information management is a system that incorporates all the processes needed to effectively manage data — both incoming and outgoing patient information. The information management system may be entirely paper-based, computer-based, or a combination of both. Whatever technology is employed, information management is another of the essentials of a quality system. This information, especially test results, are the final product of the laboratory. Laboratory directors need to ensure that the laboratory has an effective information management system in place to achieve accessibility, accuracy, timeliness, security, confidentiality, and privacy of patient information.
When planning and developing an information management system, here are some important elements to consider:
- Unique identifiers for patients and samples
- Standardized test request forms (requisitions)
- Logs and worksheets
- Processes to assure accuracy of data recording and transmission
- Protection against loss of data
- Protection of patient confidentiality and privacy
- Effective reporting systems
- Effective and timely communication
8. Occurrence management
An occurrence (or “incident”) is any event that has a negative impact on the laboratory, including its patients, personnel, equipment, or the environment in which it operates. All such events must be addressed in an occurrence management program. Occurrence management (also known as “Incidence management”) is a central part of continuous quality improvement (CQI) — it is the process by which errors are identified and handled.
The goal of an occurrence management program is to correct errors that have occurred in testing or communication, and to change the process so that the error is unlikely to occur again. Well-managed laboratories will also review their systems and detect process problems that could possibly cause errors at some time in the future, allowing for prevention of these errors. Occurrence management requires a process for detecting and documenting these occurrences, for handling them properly, and for taking corrective action to reduce the chance of recurrence.
Common errors include the following:
- Patient identification error
- Specimen misplacement
- Specimen transport delayed and proper temperature not maintained
- Specimen not kept in the right environment to maintain its integrity
- Contaminated specimens
- Performing an inappropriate test
- Performing a test inconsistent with the written procedure
- Lack of QC/QA
- Transcription and clerical errors
Occurrences are detected through various means, such as supervisory review, physicians’ or patients’ complaints, QC/QA results, or findings from external audits. Immediate remedial corrective action should be undertaken before the result is reported. Ultimately, corrective actions should be implemented to prevent similar errors from recurring.
9. Assessment
Assessment is a tool for examining laboratory performance and comparing it to known standards or to performance of other laboratories. Assessment may be internal, performed by the laboratory’s own staff, or it may be external, conducted by an external group or agency outside the laboratory.
Internal quality assessments can be conducted by the staff of the laboratory to identify weaknesses and undertake corrective actions. Quality indicators can be defined by the laboratory management and staff to complement the use of internal QC. While internal QC primarily assesses the examination steps, quality indicators can be designed to monitor the pre- and post-examination steps. Quality indicators are addressed in ISO 9001 and ISO 15189 documents and are used to determine how well an organization meets operational and performance expectations.
ISO 9001 requires that quality objectives be measurable and requires collecting and analyzing specific information or data upon which one can determine effectiveness of processes and continual improvement. Thus, the objectives or indicators must be quantifiable or otherwise capable of analysis, allowing for an assessment of the success of the quality system.
ISO 15189 requires the laboratory to implement quality indicators to systematically monitor and evaluate the laboratory’s contribution to patient care, including outcomes. Also, laboratory management must ensure that the medical laboratory participates in quality improvement activities. Quality indicators measure data through the following:
- Provide information about the performance of a process.
- Determine quality of services.
- Highlight potential quality concerns.
- Identify areas that need further study and investigation.
- Track changes over time.
External quality assessment (EQA) is a system for objectively checking the laboratory’s performance using an external agency or facility. There are three commonly used EQA methods or processes:
- Proficiency testing (PT) is performed through a set of unknown specimens sent regularly to the laboratory by a PT provider. The laboratory reports the results back to the PT provider who compares the test results with peer determined results and grades a pass (all results concordant) or fail (any discrepant results) for the PT.
- Confirmation by sending split samples to a reference laboratory for testing and comparing the results.
- On-site visits conducted by governmental, certification, or accreditation bodies.
The value of a well-designed assessment is that it will reveal weaknesses in the pre-analytical, analytical, and post-analytical phases. Information is gathered about:
- Processes and operating procedures
- Staff competence and training
- Equipment
- Environment
- Handling of samples
- Quality control and verification of results
- Recording and reporting practices
The findings are compared with the laboratory’s internal policies and to a standard or external benchmark. Any breakdown in the system or departure from procedures will be identified.
10. Customer service
Customer satisfaction is a major component of a quality management system. Medical laboratories have a range of customers including patients, physicians, public health agencies, and the community. It is the responsibility of the laboratory director to ensure that customers’ needs are met.
The quality manager is responsible for measuring the degree of customer satisfaction, using surveys, indicators, and audits to take preventive and corrective action. All laboratory staff must understand the importance of customer satisfaction. Laboratory personnel must always interact with customers in a way that is appropriate, provides needed information, and is courteous.
The initial request for service originates with the physician or healthcare provider. It is important to note that in a hospital setting, the healthcare provider will be assisted by many other people, including nurses, medical assistants, phlebotomists, and secretaries or clerks. These vital hospital personnel should also be considered clients of the laboratory, and their needs must be addressed.
Another important client for the laboratory is the patient, usually including their family. Family members may play a very important role in patient management, and may help with sample collection and transport.
In many situations, laboratory tests can be ordered by the patient directly without referral from a physician or nurse. Some patients do not have the knowledge or expertise to order the right test or to interpret results. Laboratory personnel may have to provide assistance in test selection and interpretation.
11. Process improvement
The primary goal of a quality system is continuous improvement of the laboratory processes in a systematic manner. A number of tools have been described above to identify errors, such as customer service surveys, internal QC, EQA, auditing, and quality indicators. Rigorous analysis of all these tools should lead to improvements in procedures and practices. These changes should be recorded and reflected in the SOPs and implemented in the laboratory. Open communication among staff members is also important to encourage suggestions that may improve the quality and efficiency of the laboratory.
12. Facilities and safety
The laboratory should develop SOPs for biosafety, basic safe operating procedures, and waste management that are adapted to their specific role in the laboratory and in conjunction with institutional policies. The development of safety protocols and organizing appropriate safety training is the responsibility of the laboratory director, but may be delegated to the quality manager. The steps for designing a safety management program include the following:
- Developing policies and procedures for safety and biosafety in the laboratory
- Organizing safety training that teach staff to be aware of potential hazards and how to apply safety practices and techniques. The training should include information about universal precautions, infection control, chemical and radiation safety, how to use personal protective equipment (PPE), how to dispose of hazardous waste, and what to do in case of emergencies
- Setting up a process to conduct risk assessments — this process should include initial risk assessments, as well as ongoing laboratory safety audits to look for potential safety problems
Creating a Culture of Quality
Creating and maintaining a quality laboratory operation means that the dynamics of teamwork, trust, transparency, awareness, and experience work in sync to create an environment where unbiased assessments of laboratory operations can take place, resulting in the efficient implementation of necessary changes. The key to developing and maintaining this environment is the empowerment of the staff to make decisions at the level nearest to where the work is performed. It is through the buy-in of all involved, that the goals of better patient care, efficient laboratory operation, and improved staff morale are achieved.6
REFERENCES
1. Cola.org. Accessed March 17, 2023. https://www.cola.org/wp-content/uploads/2019/05/Insights2019SpringEdition.pdf.
2. Rothenberg I. IQCP: Bridging QC and QA. Lab Testing Matters. Published May 31, 2016. Accessed March 17, 2023. http://www.labtestingmatters.org/from-the-bench/iqcp-bridging-qc-and-qa/.
3. Rothenberg I. Adapting to Change: How Resilient is your Laboratory? Physiciansofficeresource.com. Published January 19, 2019. Accessed March 17, 2023. https://www.physiciansofficeresource.com/articles/laboratory/adapting-to-change-how-resilient-is-your-laboratory/.
4. Laboratory quality management system: handbook. Who.int. Published January 1, 2011. Accessed March 17, 2023. https://www.who.int/publications/i/item/9789241548274.
5. Cdc.gov. Accessed March 17, 2023. https://www.cdc.gov/meningitis/lab-manual/chpt13-quality-control-assurance.pdf#:~:text=Quality%20Control%2FQuality%20Assurance%20%28QC%2FQA%29%20can%20be%20defined%20as,laboratory%20are%20as%20correct%20and%20accurate%20as%20possible.
6. Mlo-online.com. Accessed March 20, 2023. https://www.mlo-online.com/home/article/13008807/continuous-quality-management-in-the-laboratory