To take the test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Discuss the technologies and advancements of today’s automated hematology instruments.
2. List the platelet indices that are useful in understanding certain disease states.
3. Describe the useful platelet indices in cancer, circulatory, and thrombotic diseases.
4. Describe the useful platelet indices in inflammatory concerns, metabolic concerns, infectious diseases, and other conditions.
The complete blood count (CBC) is a standard laboratory test performed routinely. The presence of quantitative changes in the number of blood cells and the related morphologic alterations serve as a basis for diagnosis and performing additional laboratory tests and other diagnostic assessments. Today’s automated hematology instruments provide a quick and accurate way to achieve these results by incorporating state-of-the-art technology of electrical impedance, conductivity, light or fluorescence absorption, light scatter, and cytochemistry.13,21,33,52 With these technologies, the CBC has grown from measurements of routine cell counts to performing leukocyte differentials, reticulocyte counts and indices, and platelet counts and indices. More recently, platelet indices have been examined and found to be a potentially useful tool that may provide direct and/or indirect diagnostic information, prognosis, or disease management.11,13
Platelets and platelet indices
The bone marrow produces megakaryocytes that release 1,500–2,000 cytoplasmic fragments of about 3–5 µm in diameter and a volume of 4.5–11 fL. These fragments are platelets that circulate in the blood for about 7–10 days and consist of a complex of various granules, secretory vesicles, and a membranous system. Platelet enumeration and morphologic evaluation have long been a traditional part of the CBC. In a normal, healthy adult, there are 150–450 x 109 platelets/L, with a total blood volume of approximately one trillion platelets and one-third residing in the spleen.9,59 Platelet counts (PLC) are a known factor in response to various disease states. Activated platelets play a significant role in hemostasis by interacting at vascular injury sites, releasing phospholipids that activate coagulation factors. More recently, platelets have been associated with acute phase reactants in response to inflammatory conditions and can influence other cell types in dealing with various pathophysiology situations.10 These changes in platelet morphology and proliferation kinetics are reflected in platelet indices and are now thought to be useful as a set of biomarkers for understanding and managing certain diseases or conditions. Platelet indices include the following parameters:
- Mean platelet volume (MPV): The average peripheral blood platelet size.
- Platelet Distribution Width (PDW): Reflects platelet anisocytosis, a function of platelet activation when platelets change in size and morphology.
- Platelet large cell ratio (P-LCR): The percentage of platelets that are larger than 12 fL and are metabolically and enzymatically more active.
- Immature Platelet Fraction (%IPF): Measures the percentage of young, immature platelets present, a test similar to what the reticulocyte count is to red blood cells. The %IPF often increases as the platelet count increases reflecting the early release of immature platelets (reticulated platelets) from the bone marrow. A decreased %IPF result is generally due to bone marrow suppression.
Plateletcrit (PCT):Calculated as ‘platelet count x MPV/10,000.’ It is reported as a percentage reflecting the total volume occupied by platelets in peripheral blood. Similar to the PCT is the platelet mass index (PMI), calculated as the ‘platelet count x (MPV/1000) fl/nL’ and is thought to be a useful marker for inflammation.12,65,72
Cancer
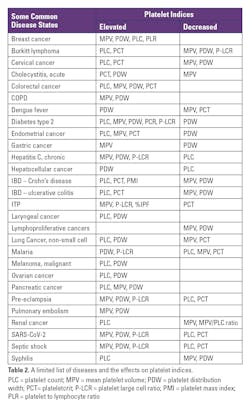
Changes in platelet count numbers can vary due to the presence of cancer cells, inflammation, or chemotherapy. Higher platelet counts as seen in breast, endometrial, ovarian, lung, colon, stomach, esophagus, renal, and kidney cancers, may reflect a more aggressive cancer.51,73,75 Circulating platelets will adhere to the vessel endothelium wall and tumor cells. As more platelets cover tumor cells, there is resistance to the shear blood flow force, which may contribute to evading the host immune response. MPV has been thought of as a surrogate marker for activated platelets generally associated with inflammatory conditions and often present in late-stage cancers. Activated platelets release microparticles containing cytokines and growth factors. It is believed that cancers can induce interleukin-6 (IL-6; a pro-inflammatory cytokine) thus stimulating the production of thrombopoietin that in turn increases platelet production.
- Breast cancer (BC): Elevated platelet counts (PLCs) have been associated with the risk of breast cancer while low PLCs were associated with a decreased risk of BC.20 In general, BC patients tend to have elevated platelet counts (PLC) and MPV levels. Those with metastases when compared to patients with locally invasive breast cancer had higher MPVs and PDWs. Two other platelet-related ratios that have also been evaluated are the MPV/P (mean platelet volume divided by the platelet count) and the PDW/P (platelet distribution width divided by the platelet count). Both of these indices were increased in patients with metastatic BC as opposed to patients with local BC, with results from both groups higher than the control group. These indices also correlated with tumor staging and grade. Another platelet-related marker that has been studied is the platelet-to-lymphocyte ratio (PLR). When elevated, it has been associated with poor overall survival and the risk of recurring breast cancer.19,63
- Gynecological cancers: It has been reported that women with ovarian cancer had elevated PLCs and a lower number of lymphocytes. In addition, the PDW levels were increased and were associated with poor survival.49 In contrast, women with cervical cancer demonstrated lower MPV and PDW levels, yet PLCs and PCTs were elevated.61 Women with endometrial cancer also showed a decreased PDW but had a significantly increased number of platelets as well as an elevated MPV and PCT.27,29
- Colorectal cancer (CRC): CRC patients have been shown to have significantly higher platelet counts (PLC), PCT, and MPVs than normal or patients with only colon adenomas. The PDW was higher in CRC patients compared to healthy controls, but lower than what was observed in colon adenoma (CA) patients. The blood tests CEA and CA19-9 are often elevated in presence of various cancers including CRC. It has been shown that the PCT and CEA results, when taken together, were more efficient in discriminating CRC patients from CA patients.74 Further, patients with metastases had higher MPV values when compared to those without metastases.
- Gastric Cancer: Patients with gastric cancer often show few if any symptoms before disease progression, thus early diagnosis is essential. These patients showed significantly lower PDW levels when compared to healthy controls and correlates well with the patient’s age, carcinoembryonic antigen (CEA), and tumor stage. PDW appears to be a predictive factor in noting the progression and prognosis of gastric cancer. Elevated MPV has been associated with the stage of disease and is thought to be useful in risk stratification and predicting low survival rates.12,17,35,48
- Laryngeal cancer: A common malignancy generally associated with the use of tobacco products and excessive alcohol intake. Patients with this cancer have elevated platelet counts and PDW, which were significant indicators of a poorer overall survival rate.30,73
- Hepatocellular carcinoma: These patients had high PDW levels. Patients with higher levels had a worse prognosis than those with a lower PDW.75 Hepatocellular carcinoma patients with thrombocytopenia and an elevated MPV had a longer overall survival rate. Further, thrombocytopenia in patients with cirrhosis has been associated with morbidity and mortality and is thought to be a risk factor for hepatocellular carcinoma.55
- Lymphoproliferative cancers: A low MPV has been reported for patients with diffuse large B-cell lymphoma and may reflect a poor prognosis. With Burkitt’s lymphoma, PLC and PCT were elevated while MPV, PDW, and P-LCR were decreased.6 CLL patients with a lower MPV before starting treatment had a worse prognosis.37 Patients with acute lymphoblastic leukemia (ALL) had lower PDW levels at diagnosis than at remission. There were no significant differences in MPV and PDW when ALL patients were compared to acute myeloid leukemia patients (AML).1
- Malignant Melanoma (MM): MM patients with thrombocytosis have been shown to have metastasis and shorter overall survival. An elevated PDW also reflects a poor prognosis and has been suggested as an independent prognostic factor in the overall survival of melanoma patients. Higher MPV levels were seen in patients with basal and squamous cell carcinomas when compared to healthy controls, yet MPV was not elevated in MM.15,30,51
- Non-small cell lung cancer: These patients showed significantly lower MPV and PCT values than the control group, but PDW and platelet counts (PLC) were elevated. PLCs and PCTs were seen to be higher in metastatic patients than for those without metastases.12,36,47,48
- Pancreatic cancer: An aggressive cancer with a poor prognosis often presenting with few if any, symptoms. The cancer markers CA19-9, CA125, and CEA are elevated generally indicating the presence of cancer but are not cancer specific. Studies have shown that the PLC, MPV, and PDW levels are also elevated in these patients compared to control groups. The PDW showed similar sensitivity and specificity with CA19-9 values suggesting that elevated CA19-9 and PDW levels may serve as significant indicators of pancreatic cancer.34,48,67
- Renal cell carcinoma (RCC): RCC studies have shown that thrombocytosis may indicate a poor prognosis for certain cancers. It has also been reported that survival times were shorter for RCC patients with low MPVs when compared to normal MPVs, thus serving as a potential prognosis predictor. Further, patients with a low MPV had a worse five-year overall survival than those with higher MPV levels and a greater risk of death. When evaluating the MPV/PLC ratio, decreased values projected a higher risk of disease progression and poor overall survival.31,36,71
Circulatory concerns
According to the Centers for Disease Control and Prevention (CDC), one person dies from cardiovascular disease (CVD) every 34 seconds in the United States each year. CVDs are a group of disorders presenting as four main types: coronary heart disease, stroke, peripheral arterial disease, and aortic disease. It is well known that platelets play a pivotal role in CVDs with the secretion of substances that enhance coagulation, inflammation, thrombosis, and arteriosclerosis.
- Acute myocardial infarction (AMI) patients had an elevated MPV when compared to non-AMI patients and have been associated with increased mortality following an MI or with angioplasty with recurring stenosis. It has been suggested that the MPV may serve as a useful prognosticator in patients with CVD and has been linked to other cardiovascular risk factors such as smoking, diabetes, obesity, hypertension, and hyperlipidemia.14
- Acute mesenteric ischemia is a syndrome due to an embolism or mesenteric venous thrombosis resulting in narrowing or blocked blood vessels. The MPV is significantly higher in such patients and may serve as a prognostic indicator. Those patients with the highest MPV values were less likely to survive vascular damage to the liver and kidneys.10 Acute ischemic stroke (AIS) is due to thrombotic and embolic mechanisms. AIS patients, after undergoing thrombolytic therapy, experienced higher MPV levels. In one study, there was a correlation between worse outcomes at three months and elevated MPV. Lower MPV levels at hospital admission predicted a good outcome.70
- Pulmonary embolism (PE) occurs when a blood clot (usually from the leg) travels to a lung artery. Traditionally, clinical manifestations of a pulmonary embolism may be non-specific and are the third most common cause of cardiovascular death. These patients demonstrate higher MPV and PDW values. Studies have shown there is a positive correlation between MPV and the increased risk of PE. Platelet counts, while in the normal range, tended to be significantly lower in the more severe PE cases than in other cases. Elevated MPV and PDW combined with the D-Dimer test provide a strong indication of pulmonary embolism.23,32
Other thrombotic-related concerns
Other thrombotic concerns include thrombocytosis and thrombocytopenia and have been associated with various medical issues as potential risk factors. In one study of surgical patients, those with thrombocytosis (platelet counts > 450 x 109/L) had a high number of post-operative issues such as failed wound healing, increased hospital readmissions, and return to the operating room, while those patients with thrombocytopenia (platelet counts below 150 x 109/L) were at risk with minor complications, post-operative anemia requiring transfusion and some with cardiac events.28 The presence of thrombocytosis and IL-6 reflect an inflammatory response that often accompanies many cancers. Hypercoagulability and malignancies can result in thromboembolisms a common cause of death in cancer patients.12
Thrombocytopenia is due to either 1) platelet destruction/consumption as seen in immune thrombocytopenic purpura (ITP), disseminated intravascular coagulation (DIC), thrombotic thrombocytopenic purpura (TTP); or 2) hypoproductive thrombocytopenias with outright bone marrow failure generally due to chemotherapy-induced bone marrow toxicity.
- ITP is an autoimmune problem that attacks and destroys platelets in error. In hyperdestructive/consumptive thrombocytopenia, the MPV, P-LCR, and %IPF are elevated and significantly higher than in patients with hypoproductive thrombocytopenia. As expected, the plateletcrit (PCT) is low due to significantly fewer platelets.24
- Differentiating ITP from hypoproductive thrombocytopenia may avoid the need for a bone marrow aspiration.43,44 In evaluating PIs, the immature platelet fraction (%IPF) values were particularly high in those patients with hyperdestructive/consumptive thrombocytopenia when compared to those with hypoproductive thrombocytopenia.24 When compared to controls and patients with myelodysplastic syndrome (a hypoproductive thrombocytopenic condition), ITP patients had significantly lower PLCs and a lower PCT. However, the ITP patients had higher MPV levels than the control group. The PDW for the ITP patients was lower than that of the MDS group, but still significantly higher than the controls.60 In a similar study, the MPV, PDW, and P-LCR values were significantly higher in ITP patients than in those with hypoproductive thrombocytopenia. In general, MPV, PDW, P-LCR, and %IPF levels tend to be elevated in ITP patients when compared to healthy controls, yet the hypoproductive thrombocytopenia patients did not show this.44
Inflammatory-related concerns
Inflammatory conditions can initiate platelet activation causing morphological changes in the platelets such as swelling and pseudopodia (platelet anisocytosis) and may mark activation of aggregation activity. These changes are usually reflected in increased MPV and PDW.
- Cholecystitis most often occurs when the cystic duct of the gallbladder is blocked resulting in the accumulation of gallstones and is typically diagnosed using ultrasonography in addition to other laboratory tests (CRP, ESR, and WBC). Patients with acute cholecystitis have been shown to have significantly lower MPV values yet their PDW and PCT values were significantly higher when compared to a control group, thus these tests may be of value in the early diagnosis of cholecystitis.10
- Inflammatory Bowel Disease (IBD) includes ulcerative colitis (UC) and Crohn’s disease (CD). Patients with IBD can develop arterial and venous thrombotic events, ultimately resulting in platelet activation. Active UC patients tend to have significantly higher PLCs but lower PDW values when compared to inactive UC patients and controls. The PCT was also significantly higher while the MPV was lower. Crohn’s Disease (CD) patients have higher PLCs, yet significantly lower PDW values when compared to controls. These patients also had an increased plateletcrit (PCT) and platelet mass index (PMI) compared to the controls. When comparing the CD group with the UC group, the PDW values were significantly lower for the CD group. The MPV was lower in the UC and CD groups compared to the controls. Further, active UC (AUC) patients showed significantly higher PLC and lower PDW when compared to inactive UC patients. When AUC patients were compared to healthy subjects, PCTs were significantly higher while the MPV was significantly lower. Active CD patients showed significantly elevated PLC, PCT, and PMI mean values than inactive CD patients and healthy controls.18
Metabolic concerns
- Diabetes affects 34.2 million people in the United States, with up to 88 million in the pre-diabetic stage according to the CDC. Type II diabetes is characterized by hyperglycemia, hypertension, dyslipidemia, impaired fibrinolysis, and elevated pro-coagulation factors. These factors contribute to greater platelet activity and potential diabetic angiopathy. Studies have shown that the PCT may be only slightly higher, but the MPV, PDW, PLC, and P-LCR tend to be elevated compared to non-diabetic individuals.46,58,69 PIs have also been shown to correlate with hemoglobin A1c levels.46 In these studies, the PDW was significantly higher in diabetics with complications than those without. Thus, platelet indices may be useful in predicting and monitoring diabetic patients.
Infectious diseases
Infectious diseases can cause hematological changes including platelets, which have historically been associated with coagulation and hemostasis. Acute infections can inhibit megakaryopoiesis, however, chronic infections tend to stimulate the production of inflammatory cytokines (IL-1, IL-6, and TNF alpha), which in turn activate megakaryopoiesis, resulting in the early release of large, young platelets.
- Chronic hepatitis C virus (HCV) patients often develop hepatic fibrosis, cirrhosis, and complications of end-stage liver disease. Platelet parameters from these patients have been shown to correlate with the stage of the disease. Patients with advanced fibrosis demonstrated significantly lower platelet counts while the MPV, PDW, and P-LCR were all elevated.57
- Malaria is a blood parasitic disease, a result of protozoan infection via a mosquito vector. A decreased platelet count is the most frequent complication of malaria most likely due to sequestration. The PLC, MPV, and PCT are decreased when compared to healthy adults. The PDW and P-LCR are increased when compared to healthy individuals. It has been reported that the P-LCR and PCT are sensitive and specific markers in the diagnosis and prognosis of severe malaria infections. As the PLC and PCT decrease, increased levels of parasitemia are observed thus they may serve as useful markers for disease progression. Because these changes in platelet parameters are thought to reflect higher levels of parasitemia, platelet degranulation occurs resulting in the release of platelet factor 4, which is thought to kill malarial parasites.7,61
- Dengue fever is a mosquito-borne viral disease that is generally found mostly in tropical/subtropical areas. Along with typical symptoms of fever, rash, muscle aches, and joint pain, patients with dengue fever may also have abnormal platelet indices. Low MPV and PCT are observed along with an elevated PDW. As with malaria, disease severity can be reflected in platelet indices changes.39
- In a syphilis study with over 2,000 patients with primary and secondary syphilis, there was significant thrombocytosis and decreased MPV and PDW when compared to healthy individuals. When treated, these abnormalities were resolved. The elevated platelet count was shown to correlate with other syphilis markers such as RPR, cerebral spinal fluid-WBC, CSF-protein, and CSF-VDRL. In contrast, decreased MPV and PDW were negatively associated with these parameters.25
- SARS-CoV-2 (COVID-19) studies have indicated a relationship between platelet indices and COVID-19-positive patients. In critically ill COVID-19 patients, elevated D-dimers were present along with a prolonged prothrombin time (PT) and a modest decrease in platelets. Viral infections have been known to cause platelet activation thus releasing chemokines, which cause changes in platelet indices. COVID-19 patients showed significantly higher MPV, P-LCR, and PDW levels when compared to COVID-19-negative patients. Further, platelet counts (PLCs) and plateletcrits (PCTs) were lower than those of COVID-19-negative patients, though not at a significant level. In another study with COVID-positive children, the PCT was lower when compared to other respiratory infections, though again, not at a significant level. In this same group, the MPV and the PDW were significantly higher than in the control group.16 A small number of COVID-positive patients showed an increased %IPF when compared to normal. In another study, the platelet-to-lymphocyte ratio (PLR) was shown to be elevated.40,50
- Septic Shock is a life-threatening condition when blood pressure drops precipitously after a bacterial, fungal, or viral infection. Studies have shown that patients with septic shock who did not survive had increasing MPV, PDW, and P-LCR levels while the PLC and PCT were decreased. P-LCR correlated to the MPV in septic shock patients. The bone marrow may release younger, larger platelets due to stress-induced platelet destruction, which also results in decreased PLCs.19,56
Other Concerns
- Pre-eclampsia (PE) is a serious complication in 3–8% of pregnancies characterized by high blood pressure and proteinuria often leading to maternal morbidity and even mortality. In addition, 5%–10% of women with uncomplicated pregnancies may have a decreased PLC of <150,000/µL, with about 70%–80% having a continuous drop in platelets (gestational thrombocytopenia; PLC<70,000/µL). This type of thrombocytopenia is similar to ITP and is not always readily differentiated. The PCT was significantly decreased in women with severe and mild pre-eclampsia when compared to a control group. The platelet count (PLC) significantly decreases as the degree of severity of pre-eclampsia progresses.4,52,64 The MPV, PDW, and P-LCR are elevated in severe PE as opposed to mild PE and control groups. These changes in MPV and PDW correlated with increases in blood pressure and thus may be useful in the early detection of pre-eclampsia and its severity.65 In healthy individuals, the MPV and PDW are proportionally related, however, in preterm labor, the MPV has been noted to decrease as the PDW increases.48 Of interest, patients experiencing a missed abortion showed elevated PLC, PCT, and PMI, while those with recurrent miscarriages showed elevated PDW.8,66
- Chronic obstructive pulmonary disease (COPD) is an inflammatory condition that is complicated by various comorbidities including pulmonary hypertension (PH). Platelets are thought to be part of the pathogenesis and the progressive nature of COPD. Platelet activation in COPD patients results in an elevated MPV and correlates with a greater incidence of advanced disease state and was particularly higher with those patients that had PH.42 Overlap syndrome (OS) involves the existence of COPD and obstructive sleep apnea syndrome causing platelet activation. Subsequently, both the MPV and PDW are elevated in such patients when compared to healthy controls.5
Discussion
Platelets play an important part in coagulation and hemostasis but also in inflammation, immunity, malignancy, and organ regeneration.55 Platelet counts certainly provide significant information in the diagnosis and treatment of patients, advances in technology have allowed for closer scrutiny revealing other associated properties of platelets. As platelet numbers increase or decrease as a function of a clinical condition and when activated, platelet indices may also play a part in supporting a diagnosis or prognosis. For example, the PDW may be a more sensitive indicator of platelet activation than the MPV, yet together, as been shown, they can serve as indicators of patient morbidity and mortality.12 Of interest, elevated plateletcrits (PCTs) have been noted to appear three or more years prior to the diagnosis for some cancers, and for lung cancer, up to ten years before diagnosis.20,31,36
Platelets normally circulate in a resting state but upon stimulation, become activated. When activated, conformational changes within platelet glycoprotein IIb/IIIa promote fibrinogen binding that then acts as a bridge for platelet-to-platelet contact resulting in aggregation. Platelet surfaces have receptors for adhesive glycoproteins and are integral in maintaining healthy hemostasis by adhering to damaged blood vessels, aggregating, and enhancing thrombin production. Stimulated platelets undergo morphologic changes, swell, and degranulate. Measurements of platelets that undergo these various changes are reflected in platelet indices (PI).
Platelet indices are often part of an automated CBC, thus readily available and inexpensive to obtain. Table 3 lists some hematology analyzers that can evaluate various platelet indices. As with other laboratory measurements, variation of normal PI intervals may be dependent on race, age, gender, smoking, alcohol consumption, and physical activity requiring each laboratory to establish reference ranges. Other considerations when measuring platelets and platelet indices are the presence of other cell types, cell fragments, platelet clumps, improper detection of large platelets, and micro red blood cells that may be counted as ‘large’ platelets. Because platelets are fragile, blood collection utilizing minimal tourniquet pressure and maintaining the specimen at room temperature are optimal practices for platelet analyses. Any delay in analyzing the specimen (must be processed within 120 minutes) can affect changes. The type of anticoagulant used to collect blood may also have an effect.68 In addition, due to variations in the analytical methodology used, there is also a need to establish harmonization and standardization to ensure accurate data interpretation that can be applied to clinical relevance.11,48,54Chronic infections can stimulate cytokine (IL-1, IL-6, TNF alpha) production thus activating megakaryopoiesis and releasing younger and larger platelets into the blood resulting in an elevated MPV. These larger platelets (high MVP) have more granules, which are released within the first 24–36 hours, thus more metabolically active.68 In acute infections, the production of megakaryocytes can be inhibited. Often acute infections result in the destruction of large and active platelets at the inflammatory site(s), thus the MPV may be lower. These changes in the platelet indices have also been noted in inflammatory bowel disease, rheumatoid arthritis, ankylosing spondylitis, ulcerative colitis, and atherosclerosis.8,26
Breast, lung, colon, esophageal, gastric, renal transitional cell, gynecological, melanoma, and glioblastoma cancers have been associated with thrombocytosis. It has been proposed that cancer cells may activate platelets resulting in thrombocytosis. and subsequently, may play a role in promoting tumor growth, angiogenesis, metastasis, and cancer-associated thrombosis. Further, it has been suggested that anti-platelet drugs may mitigate such activities.41 As platelet numbers increase or decrease, platelet indices can also change and thus serve as indicators of disease presence or progression. For example, metastatic colorectal cancer shows significantly higher MPVs than those patients without metastatic cancer. Elevated MPV values in gastric cancer patients decreased with tumor resection. Patients with thyroid cancer had higher MPV levels than those with benign goiter or normal controls.36 Changes in the MPV have also been observed in patients with septic shock, appendicitis, pancreatitis, and infective endocarditis.19
Platelet indices in themselves, are not uniquely diagnostic but may serve as an adjunct to the early evaluation of various patient conditions. It should be noted that most of the studies cited had certain limitations such as the use of different testing methodologies or noting how blood was collected and stored. Some studies were retrospective with some conflicting reports. Nonetheless, it appears that measuring platelet indices can be useful in following patients. It is inexpensively done as part of a routine CBC and in some cases may obviate the need for further and more expensive testing. More prospective studies of platelet indices are needed to establish consistent normal reference intervals and to further explore how PIs can be used in predicting various patient outcomes including the risk of death as they relate to cancers, infections, inflammation, diabetes, thrombotic concerns, and other serious medical disorders. Identifying abnormal platelet indices could play a useful role in confirming, monitoring, and/or predicting certain disease outcomes.
References
1. Al-Sweedan S, Matalka II. Platelet indices as quality markers for remission in patients with leukemia. J Hematol. 2012;1(2-3):54-57. doi:10.4021/jh16w.
2. Ali U, Knight G, Gibbs R, Tsitsikas DA. Reference intervals for absolute and percentage immature platelet fraction using the Sysmex XN-10 automated haematology analyser in a UK population. Scand J Clin Lab Invest. 2017;77(8):658-664. doi:10.1080/00365513.2017.1394488.
3. Ali U, Gibbs R, Knight G, Tsitsikas D. Sex-divided reference intervals for mean platelet volume, platelet large cell ratio and plateletcrit using the Sysmex XN-10 automated haematology analyzer in a UK population. Hematol Transfus Cell Ther. 2019;41(2):153-157. doi:10.1016/j.htct.2018.09.005.
4. Alkholy EA, Farag EA, Behery MA, Ibrahim MM. The significance of platelet count, mean platelet volume, and platelet width distribution in preeclampsia. AAMJ. 2013;11(1).
5. Archontogeorgis K, Voulgaris A, Papanas N, et al. Mean Platelet Volume and Platelet Distribution Width in Patients With Obstructive Sleep Apnea Syndrome and Concurrent Chronic Obstructive Pulmonary Disease. Clin Appl Thromb Hemost. 2018;24(8):1216-1222. doi:10.1177/1076029618788178.
6. Asare R, Opoku-Okrah C, Danquah KO, et al. Expression of platelet parameters and platelet membrane glycoproteins in childhood Burkitt lymphoma. Leuk Res. 2019;84:106189. doi:10.1016/j.leukres.2019.106189.
7. Asmerom H, Gemechu K, Bete T, et al. Platelet Parameters and Their Correlation with Parasitemia Levels Among Malaria Infected Adult Patients at Jinella Health Center, Harar, Eastern Ethiopia: Comparative Cross-Sectional Study. J Blood Med. 2023;19;14:25-36. doi:10.2147/JBM.S394704.
8. Boshnak N, Boshnaq M, Elgohary H. Evaluation of Platelet Indices and Red Cell Distribution Width as New Biomarkers for the Diagnosis of Acute Appendicitis. J Invest Surg. 2018;31(2):121-129. doi:10.1080/08941939.2017.1284964.
9. Briggs C, Harrison P, Machin SJ. Continuing developments with the automated platelet count. Int J Lab Hematol. 2007;29(2):77-91. doi:10.1111/j.1751-553X.2007.00909.x.
10. Budak YU, Polat M, Huysal K. The use of platelet indices, plateletcrit, mean platelet volume and platelet distribution width in emergency non-traumatic abdominal surgery: a systematic review. Biochem Med (Zagreb). 2016;26(2):178-93. doi:10.11613/BM.2016.020.
11. Buoro S, Seghezzi M, Manenti B, et al. Biological variation of platelet parameters determined by the Sysmex XN hematology analyzer. Clin Chim Acta. 2017;470:125-132. doi:10.1016/j.cca.2017.05.004.
12. Cheng S, Han F, Wang Y, et al. The red distribution width and the platelet distribution width as prognostic predictors in gastric cancer. BMC Gastroenterol. 2017;20;17(1):163. doi:10.1186/s12876-017-0685-7.
13. Chhabra G. Automated hematology analyzers: Recent trends and applications. J Lab Physicians. 2018;10(1):15-16. doi:10.4103/JLP.JLP_124_17.
14. Chu SG, Becker RC, Berger PB, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8(1):148-56. doi:10.1111/j.1538-7836.2009.03584.x.
15. Cihan YB, Baykan H. Comparison of platelet counts and mean platelet volume levels in skin cancer patients and healthy individuals. Austinpublishinggroup.com. Accessed August 18, 2023. https://austinpublishinggroup.com/disease-markers/fulltext/jdm-v2-id1034.php
16. Dobrijević D, Antić J, Rakić G, et al. Could platelet indices have diagnostic properties in children with COVID-19? J Clin Lab Anal. 2022;36(12):e24749. doi:10.1002/jcla.24749.
17. Zarad M, El Lehleh A, El-Abd N, Gohar S. Diagnostic value of platelet indices, carbohydrate antigen 19-9 and carcinoembryonic antigen in differentiating malignant from benign gastric ulcers. Menoufia Med J. 2019;32(4):1452. doi:10.4103/mmj.mmj_186_19.
18. Galijašević M, Dervišević A, Fajkić A, Avdagić N, Suljević D. Platelet Mass Index and Other Platelet Parameters in the Assessment of Inflammatory Bowel Diseases Activity. Curr Health Sci J. 2021;47(4):566-574. doi:10.12865/CHSJ.47.04.13.
19. Gao Y, Li Y, Yu X, et al. The impact of various platelet indices as prognostic markers of septic shock. PLoS One. 2014;13;9(8):e103761. doi:10.1371/journal.pone.0103761.
20. Giannakeas V, Kotsopoulos J, Cheung MC, et. Al. Analysis of platelet count and new cancer diagnosis over a 10-year period. doi:10.1001/jamanetworkopen.2021.41633.
21. Giovanetti TV, do Nascimento A, de Paula JP. Platelet indices: Laboratory and clinical applications. Rev Bras Hematol Hemoter. 2011;33(2):164-5. doi:10.5581/1516-8484.20110040.
22. Guo W, Lu, X, Liu Q, et al. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: An updated meta-analysis of 17079 individuals. Cancer Med. 2019;8(9):4135-4148. doi:10.1002/cam4.2281.
23. Huang J, Chen Y, Cai Z, Chen P. Diagnostic value of platelet indices for pulmonary embolism. Am J Emerg Med. 2015;33(6):760-3. doi:10.1016/j.ajem.2015.02.043.
24. Jeon K, Kim M, Lee J, et al. Immature platelet fraction: A useful marker for identifying the cause of thrombocytopenia and predicting platelet recovery. Medicine (Baltimore). 2020;99(7):e19096. doi:10.1097/MD.0000000000019096.
25. Jiang N, Ye M, Yan J, et al. Platelet indices are the promising biomarkers in monitoring disease activities in patients with syphilis. Int J Infect Dis. 2022;118:230-235. doi:10.1016/j.ijid.2022.03.014.
26. Joergensen MK, Bathum L. Reference intervals for mean platelet volume and immature plate fraction determined on a Sysmex XE5000 hematology analyzer. Scand J Clin Lab Invest. 2016;76(2):172-6. doi:10.3109/00365513.2015.1124448.
27. Karateke A, Kaplanoglu M, Baloglu A. Relations of Platelet Indices with Endometrial Hyperplasia and Endometrial Cancer. Asian Pac J Cancer Prev. 2015;16(12):4905-8. doi:10.7314/apjcp.2015.16.12.4905.
28. Kim M, Ling K, Nazemi A, et al. Abnormal preoperative platelet count may predict postoperative complications following shoulder arthroplasty. JSES Int. 2022 ;19;6(6):935-941. doi:10.1016/j.jseint.2022.06.008.
29. Kurtoglu E, Kokcu A, Celik H, et al. Platelet indices may be useful in discrimination of benign and malign endometrial lesions, and early and advanced stage endometrial cancer. Asian Pac J Cancer Prev. 2015;16(13):5397-400. doi:10.7314/apjcp.2015.16.13.5397.
30. Li N, Diao Z, Huang X, et al. Increased platelet distribution width predicts poor prognosis in melanoma patients. Sci Rep. 2017;7;7(1):2970. doi:10.1038/s41598-017-03212-y.
31. Lin YC, Jan HC, Ou HY, et al. Low preoperative mean platelet volume/platelet count ratio indicates worse prognosis in non-metastatic renal cell carcinoma. J Clin Med. 2021;19;10(16):3676. doi:10.3390/jcm10163676.
32. Lipinska A, Ledakowicz-Polak A, Krauza G, et al. Complex calculation or quick glance? Mean platelet volume – new predictive marker for pulmonary embolism. Ther Clin Risk Manag. 2018;9;14:2221-2228. doi:10.2147/TCRM.S181381.
33. Macey MG, Carty E, Webb L, et al. Use of mean platelet component to measure platelet activation on ADVIA 120 haematology system. Cytometry. 1999;15;38(5):250-5. doi:10.1002/(sici)1097-0320(19991015)38:5<250::aid-cyto8>3.3.co;2-b.
34. Mai S, Inkielewicz-Stepniak I. Pancreatic cancer and platelets crosstalk: A potential biomarker and target. Front Cell Dev Biol. 2021;10;9:749689. doi:10.3389/fcell.2021.749689.
35. K V M, Jonnada P, N SK, Anwar A. Role of Mean Platelet Volume in the Prognosis of Locally Advanced Gastric Cancer: A Tertiary Cancer Center Experience. Cureus. 2020;10;12(7):e9109. doi:10.7759/cureus.9109.
36. Masternak M, Knap J, Giannopoulos K. The prognostic value of mean platelet volume in cancer patients. Acta Haematol Pol. 2019;50(3):154-158. doi:10.2478/ahp-2019-0025.
37. Masternak M, Pula B, Knap J, et al. Mean platelet volume has prognostic value in chronic lymphocytic leukemia. Cancer Manag Res. 2020;12;12:9977-9985. doi:10.2147/CMAR.S246385.
38. Masutani R, Ikemoto T, Maki A, et al. Mean platelet component and mean platelet volume as useful screening markers for myelodysplastic syndrome. Health Sci Rep. 2018;2;1(5):e50. doi:10.1002/hsr2.50.
39. Meena VK, Bihari S, Meena SR. Diagnostic significance of platelet indices in dengue fever in an endemic area. Internat J Res Rev. 2020;7(2):315-319.
40. Mezgebe M, Jacobson BF, Mayne ES, Louw S. Change in platelet indices in patients with Coronavirus disease-2019 (COVID-19): A reflection of platelet activation and contribution to immunothrombosis? Int J Lab Hematol. 2022;44(1):e46-e48. doi:10.1111/ijlh.13705.
41. Mezouar S, Frere C, Darbousset R, et al. Role of platelets in cancer and cancer-associated thrombosis: Experimental and clinical evidences. Thromb Res. 2016;139:65-76. doi:10.1016/j.thromres.2016.01.006.
42. Mohamed MF, Ali A, Abbas A, et al. Mean platelet volume as a predictor of pulmonary hypertension in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2019;23;14:1099-1108. doi:10.2147/COPD.S176413.
43. Mowfay NM, Elkeiy M, Khedr AH. Role of platelet indices and antiplatelet antibody in differentiating immune thrombocytopenic purpura from other causes of thrombocytopenia. Egypt J Hosp Med. 2019;74(8):1732-1736.
44. Nakadate H, Matsumoto K. Use of platelet indices for the differential diagnosis of pediatric immune throbocytopenia purpura (ITP). Blood. 2016;128(22).
45. Negash M, Tsegaye A, G/Medhin A. Diagnostic predictive value of platelet indices for discriminating hypo productive versus immune thrombocytopenia purpura in patients attending a tertiary care teaching hospital in Addis Ababa, Ethiopia. BMC Hematol. 2016;1;16:18. doi:10.1186/s12878-016-0057-5.
46. Nirangjhana S. Platelet indices as a tool in assessing the progression of Type 2 diabetes mellitus. Interna J Sci Res. 2020;9(1):46-48.
47. Oncel M, Kiyici A, Oncel M, et al. Evaluation of platelet indices in lung cancer patients. Asian Pac J Cancer Prev. 2015;16(17):7599-602. doi:10.7314/apjcp.2015.16.17.7599.
48. Pogorzelska K, Krętowska A, Krawczuk-Rybak M, Sawicka-Żukowska M. Characteristics of platelet indices and their prognostic significance in selected medical condition - a systematic review. Adv Med Sci. 2020;65(2):310-315. doi:10.1016/j.advms.2020.05.002.
49. Qin L, Li JY, Huang WJ, Zhang ML, Wang RT, Shen W. Higher platelet distribution width is associated with unfavorable prognosis in ovarian cancer. Cancer Biomark. 2020;28(3):365-370. doi:10.3233/CBM-191190.
50. Qu R, Lin Y, Zhang YH, et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. 2020;92(9):1533-1541. doi:10.1002/jmv.25767.
51. Rachidi S, Li H, Wallace K, et al. Preoperative platelet counts and postoperative outcomes in cancer surgery: a multicenter, retrospective cohort study. Platelets. 2020;31(1):79-87. doi:10.1080/09537104.2019.1573977.
52. Kumar A, Singh PK. A case control study to assess the role of platelet count in the early identification of preeclampsia and eclampsia. Inter J Pharmac Clin Res. 2021;14(1):105-109.
53. Ryan DH, Kaushansky K, Lichtman MA, et al. Examination of Blood Cells. In: Williams Hematology, 9e. McGraw-Hill; 2016.
54. Santos MNND. Does gender influence reference values of platelet indices? Hematol Transfus Cell Ther. 2019;41(2):104-105. doi:10.1016/j.htct.2019.04.001.
55. Scheiner B, Kirstein M, Popp S, et al. Association of platelet count and mean platelet volume with overall survival in patients with cirrhosis and unresectable hepatocellular carcinoma. Liver Cancer. 2019;8(3):203-217. doi:10.1159/000489833.
56. Sekhar SVRR, Naidu PJ, Kumar EG, Shamili M, Rao ES. Prognostic role of platelet indices in sepsis patients. Saudi J Pathol Microbiol. 2019;04(12):898-901. doi:10.36348/sjpm.2019.v04i12.005.
57. Shao LN, Zhang ST, Wang N, et al. Platelet indices significantly correlate with liver fibrosis in HCV-infected patients. PLoS One. 2020;9;15(1):e0227544. doi:10.1371/journal.pone.0227544.
58. Shilpi K, Potekar RM. A Study of Platelet Indices in Type 2 Diabetes Mellitus Patients. Indian J Hematol Blood Transfus. 2018;34(1):115-120. doi:10.1007/s12288-017-0825-9.
59. Smyth SS, Whiteheart S, Italiano JE, Jr., et al. Platelet Morphology, Biochemistry, and Function. In: Kaushansky K, Lichtman MA, Prchal JT, Levi MM, Press OW, Burns LJ, Caligiuri M. eds. Williams Hematology, 9e. New York, NY: McGraw-Hill. (2016).
60. Tang YT, He P, Li YZ, et al. Diagnostic value of platelet indices and bone marrow megakaryocytic parameters in immune thrombocytopenic purpura. Blood Coagul Fibrinolysis. 2017;28(1):83-90. doi:10.1097/MBC.0000000000000612.
61. Tangvarasittichai O, Srikong M, Tangvarasittichai S. Platelet count and platelet indices used as potential markers for first malaria infection diagnosis. Internat J Pharma Clin Research. 2016;8(10):1454-1458.
62. Tas EE, Department of Obstetrics and Gynecology, Ankara Yildirim Beyazit University School of Medicine, Ankara, Turkey, Yegin GF, et al. Is there any correlation between preoperative platelet indices and surgical prognostic factors in patients with cervical cancer? Cyprus J Med Sci. 2019;4(1):38-42. doi:10.5152/cjms.2019.676.
63. Tera Y, Azzam H, Abousamra N, et al. Platelet activation and platelet indices as markers for disease progression in women with breast cancer: Platelets and prognosis of breast cancer. Arch Breast Canc. Published online 2022:346-353. doi:10.32768/abc.202293346-353.
64. Tesfay F, Negash M, Alemu J, et al. Role of platelet parameters in early detection and prediction of severity of preeclampsia: A comparative cross-sectional study at Ayder comprehensive specialized and Mekelle general hospitals, Mekelle, Tigray, Ethiopia. PLoS One. 2019;21;14(11):e0225536. doi:10.1371/journal.pone.0225536.
65. Thalor N, Singh K, Pujani M, et al. A correlation between platelet indices and preeclampsia. Hematol Transfus Cell Ther. 2019;41(2):129-133. doi:10.1016/j.htct.2018.08.008.
66. Uckan K, Çeleğen İ, Başkiran Y, Hanlıgil E. Can Platelet Mass Index be used as a prognostic marker in the diagnosis of missed abortion patients? East J Med. 2022;27(4):627-633. doi:10.5505/ejm.2022.45549.
67. Ulutas KT, Sarici IS, Arpaci A. Comparison of Platelet Distribution Width and CA19-9 in Resectable Pancreas Cancer. Med Arch. 2018;72(3):210-213. doi:10.5455/medarh.2018.72.210-213.
68. Vinholt PJ, Hvas AM, Nybo M. An overview of platelet indices and methods for evaluating platelet function in thrombocytopenic patients. Eur J Haematol. 2014;92(5):367-76. doi:10.1111/ejh.12262.
69. Walinjkar RS, Khadse S, Kumar S, Bawankule S, Acharya S. Platelet Indices as a Predictor of Microvascular Complications in Type 2 Diabetes. Indian J Endocrinol Metab. 2019;23(2):206-210. doi:10.4103/ijem.IJEM_13_19.
70. Yao Y, Cao X, Zou R, et al. Study on the baseline factors and platelet indices that predict outcome of acute ischemic stroke patients after thrombolytic therapy. Cerebrovasc Dis. 2022;51(3):357-364. doi:10.1159/000519705.
71. Yun ZY, Zhang X, Liu YS, et al. Lower platelet volume predicts poor prognosis in renal cell carcinoma. Sci Rep. 2017;27;7(1):6700. doi:10.1038/s41598-017-07168-x.
72. Yurekli UF, Liste U, Etunc M. Could platelet mass index (PMI) be a new prognostic biomarker for COVID-19? Ann Clin Anal Med. 2022;13(1):72-75.
73. Zhang H, Liu L, Fu S, et al. Higher platelet distribution width predicts poor prognosis in laryngeal cancer. Oncotarget. 2017;18;8(29):48138-48144. doi:10.18632/oncotarget.18306.
74. Zhu X, Cao Y, Lu P, et al. Evaluation of platelet indices as diagnostic biomarkers for colorectal cancer. Sci Rep. 2018;7;8(1):11814. doi:10.1038/s41598-018-29293-x.
75. Zuo X, Kong W, Feng L, et al. Elevated platelet distribution width predicts poor prognosis in hepatocellular carcinoma. Cancer Biomark. 2019;24(3):307-313. doi:10.3233/CBM-182076.