To take the test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Discuss the viral family, prevalence, and transmission route of cytomegalovirus (CMV).
2. List the vulnerable populations and complications of CMV transmission.
3. Describe CMV testing currently used in prenatal, fetal, and newborn testing.
4. Discuss future suggestions for the screening of newborns for CMV and its utility worldwide.
Cytomegalovirus (CMV) is a beta-herpesvirus that causes viral inclusion bodies and enlarges infected cells. It is the largest herpesvirus known to infect humans, with a seroprevalence of 60–90% worldwide. 1,2 Higher prevalence occurs in lower socioeconomic groups in developing countries. 2 In the United States, nearly one-third of children have CMV by age of five, and more than half of adults have it by the age of forty.3
CMV is transmitted from person to person by direct contact. The virus is shed in body fluids — with main transmission via saliva and urine of young children to other children or adults. Other forms of transmission include sexual contact, blood transfusions, and organ transplants. In healthy individuals, CMV infection is often asymptomatic, but it may be fatal in immunocompromised patients.4 Symptoms of CMV in mild cases are described as flu-like and include fever, sore throat, fatigue, and swollen glands. More serious cases, such as those occurring in people with weakened immune systems, exhibit symptoms affecting the eyes, lungs, liver, esophagus, stomach, and intestines.
Primary infection occurs in those who have never been infected before. As with other herpes viruses, CMV remains latent in the host after the first infection and may reactivate at a later period. Reinfection occurs when a person is infected with a different viral strain.
CMV is the leading viral cause of congenital defects. CMV can cross the placenta and infect the fetus after primary infection, reactivation, or reinfection of the mother. The transmission is most likely in women with a primary CMV infection, and the risk of transmission increases throughout the third trimester.5,6 Infection occurs in 0.5% to 2.5% of neonates, and most babies with symptoms at birth (5%) have long-term effects including sensorineural hearing loss, microcephaly, chorioretinitis, and motor disabilities.7 A large percentage of asymptomatic newborns (15%) subsequently suffer impairments, most often hearing loss.7,8 Furthermore, CMV infection is the most prevalent and dangerous opportunistic infection following solid organ transplantation (SOT) or hematopoietic stem cell transplantation (SCT) and in HIV patients.2,9 CMV infection has also been linked with atherosclerosis, glioblastoma, and other diseases.10,11
There is no vaccine available to prevent CMV infection but there are antiviral drugs to treat immunocompromised individuals. Antiviral medication may improve hearing and developmental outcomes in infants with congenital cytomegalovirus (cCMV), although severe adverse effects may occur.6 Healthy individuals recover from infection without problems and treatment is not required.12
Laboratory testing for CMV diagnosis
Detection of CMV infection is particularly important in cCMV infection (pregnant women and newborns) as well as in immunocompromised individuals (e.g., SOT, SCT and in HIV patients). Since immunocompetent individuals are not at risk for developing complications, testing is not required. As the symptoms are not specific, laboratory testing is required to diagnose CMV infection. The main laboratory methods for diagnosing CMV are serology and molecular testing. Depending on the population being tested, one method is favored over the other or a combination of both is preferred. The following sections describe when serology and/or molecular testing are best used for detection of CMV.
Serology in adults and infants older than 12 months
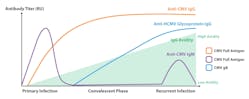
Initial infection leads to the production of CMV-specific immunoglobulin M (IgM) antibodies that persist in the blood for a short period of time and later to the production of IgG antibodies that can persist forever (Figure 1).13,14 The main challenge with IgM antibody detection is that some individuals can have persistent IgM levels for over a year.15 IgM can be also detected in reactivation or reinfection. Therefore, the detection of IgM does not confirm an active primary infection. Furthermore, IgM antibodies are not highly specific and false positive results may occur.16 A primary CMV infection can be distinguished from a past infection by measuring immunoglobulin G (IgG) seroconversion (follow-up collection samples required) and/or IgG avidity. IgG avidity tests evaluate the binding strength of IgG antibodies to the virus. Low-binding strength (low avidity) IgG antibodies are produced in response to initial CMV infection, and over the course of 2–4 months, develop into high binding strength (high avidity) (Figure 1).17 Therefore, high avidity IgG would indicate a past infection while low avidity IgG would indicate a primary infection. Due to the potential complications of CMV infection, particularly in pregnant women, is important to distinguish between primary infection, past infection, reactivation, and reinfection.
CMV testing in pregnancy
In the United States, routine screening for CMV infection during pregnancy is not recommended by the Centers for Disease Control and Prevention (CDC). The main reason is that available diagnostics cannot predict whether the fetus will be infected. Additionally, there is no vaccine available or treatment to prevent fetal infection. However, other countries, particularly Europe, recommend routine serological screening for CMV of pregnant women.18,19
Although prenatal screening is not recommended in some countries, pregnant women can consult their obstetricians and request a test to determine their CMV immune status. Typically, serological testing is offered for this purpose as a stand-alone test or as part of a (toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus) ToRCH panel.20
A combination of serological testing (IgM, IgG avidity, and IgG seroconversion) can help to discriminate between active infection, past infection, reactivation, and reinfection (Tables 1 and 2).15,21,22 In fact, the Maternal-Fetal Medicine Units (MFMU) Network from NIH provides a CMV calculator to estimate cCMV infection after maternal primary infection. These estimates are based on data from the MFMU Network Randomized Trial to Prevent Congenital Cytomegalovirus.23 This CMV calculator predicts cCMV infection in the context of primary maternal CMV infection and no ultrasonographic indications of congenital infection. Having this information may aid in patient counseling and decision making.24 This calculator considers the results of an IgM antibody test, IgG avidity, and presence or absence of virus in maternal plasma.
Serological assays using glycoprotein B (gB) as an antigen for IgG antibody detection in pregnant women are included in some guidelines for screening pregnant women in Europe.25-27 In general, the IgG antibody response to CMV gB is delayed by up to 100 days (Figure 1; Table 1).28 Therefore, IgG antibodies against gB indicate a past infection and a recent or primary infection can be excluded. These results are comparable to the finding of high avidity IgG antibodies. However, only 82% of CMV‐infected individuals produce IgG antibodies against gB.29 Consequently, a negative result can be a false-negative. Therefore, it is highly recommended to look at a combination of IgM, IgG seroconversion, IgG avidity, and antibodies against gB.Fetal population testing
When an active infection is detected in a pregnant woman, the next step is to check fetal infection. There are two prenatal tests that can be used: non-invasive (ultrasound examination) and invasive (amniocentesis). CMV isolation from amniotic fluid (amniocentesis) has been established as the gold standard due to its high sensitivity and specificity. However, amniocentesis has risks for the pregnant woman and fetus.30 On the other hand, when fetal abnormalities are detected by ultrasound and the pregnant woman has low IgG avidity antibodies, the fetus has a higher risk of being infected. Therefore, the newborn will need to be monitored to confirm or rule out cCMV infection.22
Newborn testing
Molecular testing, such as quantitative polymerase chain reaction (qPCR), is the gold standard for cCMV detection in newborns within the first 2–3 weeks of life to distinguish congenital from a postnatal infection acquired during or after delivery (Tables 1 and 2).22,31 Saliva and urine are the preferred sample types for testing because they contain high viral loads of CMV. However, blood can also be used. The CDC recommends first testing saliva and then confirming positive samples with urine because CMV is also shed in breast milk. Therefore, confirmation with urine will help to rule-out false positives from breast milk.17
If the newborn is negative, the baby is considered uninfected, and no further tests are warranted. If a newborn is infected as indicated through a positive result from molecular testing, the newborn will be monitored for hearing loss or other sequela, thus increasing opportunities for early intervention.32
Serological testing for newborns within the first 2–3 weeks is not recommended because IgM antibodies are only present in 70% of infected newborns.16 Additionally, newborn IgG antibodies mainly come from the mother and transfer through the placenta to the fetus.33 As with molecular testing, serological tests will not distinguish prenatal from perinatal CMV infection after 2–3 weeks of life.16
As mentioned above, a large percentage of infected newborns are asymptomatic at birth but develop symptoms later.7,8 Therefore, it can be helpful to screen newborns at birth. Several studies support the need of neonatal screening to identify earlier infected infants at risk to develop neurological sequelae and provide the appropriate treatment to reduce and treat CMV diseases.34 In the United States, universal screening is not included in routine newborn screening. The CDC is investigating dried blood spot (DBS) to be used for this purpose.17 This is important because DBS are collected from all newborns for metabolic screening and sometimes for detection of newborn disorders.22,35 In fact, there are already commercially available assays to detect cCMV in newborns through DBS.36 Interestingly, some states have already implemented universal screening. In February 2022, Minnesota become the first state in the nation to screen every newborn for cCMV.37
Transplantation population testing
Another population that is at risk of developing complications from CMV infection are recipients of organ or hematopoietic stem cell transplantation.
Direct detection of CMV by molecular testing is suggested for detecting and monitoring current infections in transplant recipients (Table 2). Transplant donors must be also tested for CMV active infection prior to donation.22,38 On the other hand, serological testing is recommended for transplant donors and recipients to reduce the risk of a primary infection and reactivation.38,39
Conclusion
CMV is a common virus that can infect people of all ages. As does herpes virus, CMV remains latent in the human body. Therefore, the virus can be reactivated after primary infection and induce an active infection. Immunocompromised individuals are the main population at risk to develop complications from CMV primary infection, reactivation, and reinfection. These include pregnant women, newborns, and transplant recipients. Depending on the at-risk population, either serology or molecular testing are performed to detect an active infection or differentiate a primary infection from reactivation or reinfection.
A combination of molecular testing and serology provides the most accurate diagnosis of CMV infection. Due to the complications associated with a primary infection in pregnant women, it is important to raise awareness about CMV infection and implement initiatives to reduce the risk of transmitting the virus to the fetus. Furthermore, monitoring newborns is essential for identifying the infection quickly and administering the appropriate treatment. However, in some countries, prenatal or universal newborn screening is not recommended. One of the factors influencing that decision is the cost associated with testing. Nonetheless, it would be interesting to investigate the long-term consequences and costs of not screening these two populations.
Vaccine candidates are now being evaluated in clinical studies.40 The approval of a vaccine to prevent CMV infection will have a significant impact on the groups at risk. In addition, there may be a shift in the role of serology in terms of monitoring the immune system’s response to vaccines. While waiting for a vaccine, those at risk should adopt proper hygiene practices to avoid CMV infection. For instance, frequent handwashing and avoiding touch with another person’s saliva, especially avoiding contact with the saliva and urine of small children.
References
- Wang Y-Q, Zhao X-Y. Human Cytomegalovirus Primary Infection and Reactivation: Insights From Virion-Carried Molecules. Frontiers in Microbiology. 2020;11.
- Griffiths P, Reeves M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nature Reviews Microbiology. 2021;19(12):759-773.
- CDC. About Cytomegalovirus (CMV). https://www.cdc.gov/cmv/overview.html. Published 2020. Accessed September 27, 2022.
- Gupta M, Shorman M. Cytomegalovirus. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Mack I, Burckhardt MA, Heininger U, Prüfer F, Schulzke S, Wellmann S. Symptomatic Congenital Cytomegalovirus Infection in Children of Seropositive Women. Front Pediatr. 2017;5:134.
- CDC. Congenital CMV Infection. https://www.cdc.gov/cmv/clinical/congenital-cmv.html. Accessed September 27, 2022.
- Belzile JP, Sabalza M, Craig M, Clark AE, Morello CS, Spector DH. Trehalose, an mTOR-Independent Inducer of Autophagy, Inhibits Human Cytomegalovirus Infection in Multiple Cell Types. J Virol. 2016;90(3):1259-1277.
- Goderis J, De Leenheer E, Smets K, Van Hoecke H, Keymeulen A, Dhooge I. Hearing loss and congenital CMV infection: a systematic review. Pediatrics. 2014;134(5):972-982.
- Jakharia N, Howard D, Riedel DJ. CMV Infection in Hematopoietic Stem Cell Transplantation: Prevention and Treatment Strategies. Current Treatment Options in Infectious Diseases. 2021;13(3):123-140.
- Clark AE, Sabalza M, Gordts P, Spector DH. Human Cytomegalovirus Replication Is Inhibited by the Autophagy-Inducing Compounds Trehalose and SMER28 through Distinctively Different Mechanisms. J Virol. 2018;92(6).
- Griffiths P. The direct and indirect consequences of cytomegalovirus infection and potential benefits of vaccination. Antiviral Research. 2020;176:104732.
- CDC. CMV: Clinical overview. https://www.cdc.gov/cmv/clinical/overview.html. Accessed September 27, 2022.
- Dioverti MV, Razonable RR. Cytomegalovirus. Microbiol Spectr. 2016;4(4).
- Bulka CM, Bommarito PA, Aiello AE, Fry RC. Cytomegalovirus seroprevalence, recurrence, and antibody levels: Associations with cadmium and lead exposures in the general United States population. Environ Epidemiol. 2020;4(4):e100.
- Prince HE, Lapé-Nixon MKP, New York 11754. Role of cytomegalovirus (CMV) IgG avidity testing in diagnosing primary CMV infection during pregnancy. Clin Vaccine Immunol. 2014;21(10):1377-1384.
- Bonalumi S, Trapanese A, Santamaria A, D’Emidio L, Mobili L. Cytomegalovirus infection in pregnancy: review of the literature. J Prenat Med. 2011;5(1):1-8.
- CDC. Laboratory Testing. https://www.cdc.gov/cmv/clinical/lab-tests.html. Published 2020. Accessed September 2022, 2022.
- Saldan A, Forner G, Mengoli C, Gussetti N, Palù G, Abate D. Testing for Cytomegalovirus in Pregnancy. J Clin Microbiol. 2017;55(3):693-702.
- Adler SP. Screening for cytomegalovirus during pregnancy. Infect Dis Obstet Gynecol. 2011;2011:1-9.
- Fitzpatrick D, Holmes NE, Hui L. A systematic review of maternal TORCH serology as a screen for suspected fetal infection. Prenat Diagn. 2022;42(1):87-96.
- Shimada K, Toriyabe K, Kitamura A, et al. Primary cytomegalovirus infection during pregnancy and congenital infection: a population-based, mother–child, prospective cohort study. Journal of Perinatology. 2021;41(10):2474-2481.
- Razonable RR, Inoue N, Pinninti SG, et al. Clinical Diagnostic Testing for Human Cytomegalovirus Infections. J Infect Dis. 2020;221(Suppl 1):S74-s85.
- Maternal-Fetal Medicine Units Network. CMV calculator: Congenital Cytomegalovirus Infection after Maternal Primary Infection. https://mfmunetwork.bsc.gwu.edu/web/mfmunetwork/cmv-calculator. Published 2022. Accessed September 27, 2022.
- Rouse DJ, Fette LM, Hughes BL, et al. Noninvasive Prediction of Congenital Cytomegalovirus Infection After Maternal Primary Infection. Obstetrics & Gynecology. 2022;139(3):400-406.
- Mikrobiologisch-infektiologischer-Qualitätsstandard, MiQ. MIQ issue: 35b infection immunological methods part 2. https://shop.elsevier.de/miq-heft-35b-infektionsimmunologische-methoden-teil-2-9783437415326.html. Published 2016. Accessed October 10, 2022.
- Robert Koch Institute. Cytomegalovirus Infection https://www.ki.de/DE/Content/Infekt/EpidBull/Merkblaetter/Ratgeber_Zytomegalievirus.html. Published 2014. Accessed October 10, 2022.
- Zelini P, Fornara C, Furione M, et al. Determination of anti-p52 IgM and anti-gB IgG by ELISA as a novel diagnostic tool for detection of early and late phase of primary human cytomegalovirus infections during pregnancy. J Clin Virol. 2019;120:38-43.
- Schoppel K, Kropff B, Schmidt C, Vornhagen R, Mach M. The Humoral Immune Response against Human Cytomegalovirus Is Characterized by a Delayed Synthesis of Glycoprotein-Specific Antibodies. The Journal of Infectious Diseases. 1997;175(3):533-544.
- Rothe M, Pepperl-Klindworth S, Lang D, et al. An antigen fragment encompassing the AD2 domains of glycoprotein B from two different strains is sufficient for differentiation of primary vs. recurrent human cytomegalovirus infection by ELISA. J Med Virol. 2001;65(4):719-729.
- Jummaat F, Ahmad S, Mohamed Ismail NA. 5-Year review on amniocentesis and its maternal fetal complications. Horm Mol Biol Clin Investig. 2019;40(2).
- CDC. Babies Born with Congenital Cytomegalovirus (CMV). https://www.cdc.gov/cmv/congenital-infection.html. Published 2022. Accessed September 29, 2022.
- Cannon MJ, Griffiths PD, Aston V, Rawlinson WD. Universal newborn screening for congenital CMV infection: what is the evidence of potential benefit? Rev Med Virol. 2014;24(5):291-307.
- Ciobanu AM, Dumitru AE, Gica N, Botezatu R, Peltecu G, Panaitescu AM. Benefits and Risks of IgG Transplacental Transfer. Diagnostics (Basel). 2020;10(8).
- Chiereghin A, Pavia C, Turello G, et al. Universal Newborn Screening for Congenital Cytomegalovirus Infection - From Infant to Maternal Infection: A Prospective Multicenter Study. Front Pediatr. 2022;10:909646.
- PerkinElmer. Newborn screening solutions from sample to result. https://rh.perkinelmer.com/newborn-products/#Kits. Accessed September 30, 2022.
- PerkinElmer. DETECT cCMV IN A SINGLE DRIED BLOOD SPOT PUNCH. https://perkinelmer-appliedgenomics.com/home/products/neomdx-ccmv-real-time-pcr-assay/. Accessed September 30, 2022.
- Minesotta Department of Health. Congenital cytomegalovirus approved for addition to newborn screening panel. https://www.health.state.mn.us/news/pressrel/2022/newborn020222.html. Published 2022. Accessed September 30, 2022.
- Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13512.
- CDC. Guidelines for Preventing Opportunistic Infections Among Hematopoietic Stem Cell Transplant Recipients. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr4910a1.htm. Published 2004. Accessed October 1, 2022.
- Moderna. Making Strides Toward CMV Prevention. https://www.modernatx.com/en-US/media-center/all-media/blogs/making-strides-toward-cmv-prevention. Published 2021. Accessed October 10, 2022.