To take the test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
- Describe signs and symptoms of uncomplicated influenza within patients.
- Recognize various methods for influenza testing.
- Describe the differences among testing methods.
- Identify recommended practices for storing and packing specimens.
The past two years have seen record-low flu case numbers due to social distancing and increased hygiene measures. Reduced socializing has meant less exposure and reduced herd immunity. According to the Centers for Disease Control and Prevention (CDC), reduced population immunity could result in an early and possibly severe flu season in 2022. Flu seasons varies, but influenza activity often begins to increase in October. In addition to reduced population immunity this year, flu viruses are constantly changing so it’s not unusual for new flu viruses to appear each year. In preparation for flu season, MLO has summarized CDC guidance below.
Understanding flu infection
Influenza virus is an upper respiratory infection with symptoms that include fever, chills, myalgia, headache, malaise, nonproductive cough, sore throat, and rhinitis. It is spread when infected persons cough, sneeze, or talk. People are usually infected during “flu season,” which typically occurs during the winter months, but most people recover within 3–7 days, but cough and fatigue can last up to two weeks. This is true of individuals who have uncomplicated influenza virus. About 8% of people in the United States get the flu each year, according to the CDC.
Some people who are infected with influenza can develop severe disease. Complications in young children can include otitis media, croup, bronchiolitis, tracheitis, cardiac (myocarditis and pericarditis), musculoskeletal (severe myositis), and neurologic (encephalopathy, encephalitis, transverse myelitis, and acute disseminated encephalomyelitis). Reye syndrome can be a result of influenza virus, but it has been rare since the advisory against aspirin in 1982. Influenza can also worsen medical conditions people may already have like diabetes and heart failure.
Flu testing
Testing is available to differentiate whether a person’s symptoms are the result of influenza virus or another respiratory illness. Clinicians can still diagnose flu in patients without testing, especially during flu season; however, molecular testing is recommended for all hospitalized patients with suspected influenza.1
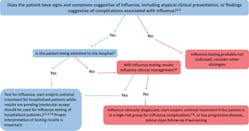
For the CDC’s guide for when to consider influenza testing, see Figure 1.2
- Confirmation of influenza virus infection by diagnostic testing is not required for decisions to prescribe antiviral medication. Decision-making should be based upon signs and symptoms consistent with influenza illness and epidemiologic factors. Initiation of empiric antiviral treatment should not be delayed while influenza testing results are pending. Antiviral treatment is clinically most beneficial when started as close to illness onset as possible. Influenza vaccine effectiveness is moderate and so a history of current season influenza vaccination does not exclude a diagnosis of influenza.
- Signs and symptoms of uncomplicated influenza vary by age, underlying health conditions, and immune function. Common signs and symptoms include fever with nonproductive cough or other suggestive respiratory symptoms, often with myalgias or headache. Fever is not always present, including in premature and young infants, immunocompromised and immunosuppressed persons, and especially in elderly persons. Note that some persons may have atypical presentations -especially infants (e.g. sepsis-like syndrome) and elderly (e.g. confusion).
- Complications associated with influenza can vary by age, immune status, and underlying medical conditions. Some examples include worsening of underlying chronic medical conditions (e.g. worsening of congestive cardiac failure; asthma exacerbation; exacerbation of chronic obstructive pulmonary disease); lower respiratory tract disease (pneumonia, bronchiolitis, croup, respiratory failure); invasive bacterial co-infection; cardiac (e.g.myocarditis); musculoskeletal (e.g. myositis, rhabdomyolysis); neurologic (e.g. encephalopathy, encephalitis); multi-organ failure (septic shock, renal failure, respiratory failure).
- Influenza testing may be used to inform decisions on use of antiviral treatment, antibiotic treatment, need for further diagnostic tests, consideration for home care, or on recommendations for ill persons living with others who are at high-risk for influenza complications. Proper interpretation of influenza testing results must consider a number of factors, including: the predictive values of the test, test sensitivity and specificity compared to a “gold standard” test, prevalence of influenza in the patient population, time from illness onset to specimen collection and whether the person may still have detectable influenza viral shedding, and source of the respiratory specimen (upper or lower respiratory tract). To maximize detection of influenza viruses, respiratory specimens should be collected as close to illness onset as possible (ideally <3-4 days after onset; molecular assays may detect influenza viral RNA in respiratory tract specimens for longer periods after illness onset than antigen detection assays). See this algorithm for more information. The Infectious Diseases Society of America (IDSA) recommends use of rapid influenza molecular assays over rapid influenza diagnostic tests (RIDTs) for detection of influenza viruses in respiratory specimens of outpatients. Consult the IDSA Influenza Clinical Practice Guidelinesexternal icon for recommendations on influenza testing and interpretation of testing results. Consult guidance on antibiotic use from the IDSA, ATS, and the AAP. Antiviral treatment is recommended as soon as possible for hospitalized patients with suspected influenza without waiting for influenza testing results of molecular assays. Guidance on antiviral treatment of influenza is available.
- All hospitalized patients with suspected influenza should be tested with molecular assays with high sensitivity and specificity (e.g. RT-PCR) since detection of influenza virus infection and prompt initiation of antiviral therapy is most clinically beneficial, and prompt implementation of infection prevention and control measures is essential for prevention of nosocomial influenza outbreaks. The Infectious Diseases Society of America (IDSA) recommends use of RT-PCR or other molecular assays for detection of influenza viruses in respiratory specimens of hospitalized patients. Consult the IDSA Influenza Clinical Practice Guidelinesexternal icon for recommendations on influenza testing and interpretation of testing results. Molecular assays can detect influenza viral nucleic acids in respiratory specimens for longer periods and with much higher accuracy than antigen detection assays. For hospitalized patients with lower respiratory tract disease and suspected influenza, lower respiratory tract specimens should be collected and tested for influenza viruses by RT-PCR because influenza viral shedding in the lower respiratory tract may be detectable for longer periods than in the upper respiratory tract, if influenza testing of upper respiratory tract specimens yields a negative result. If the patient is critically ill on invasive mechanical ventilation, and has tested negative for influenza viruses on an upper respiratory tract specimen, including by a molecular assay, a lower respiratory tract specimen (endotracheal aspirate or bronchioalveolar lavage fluid) should be collected for influenza testing by RT-PCR or other molecular assays. See Prevention Strategies for Seasonal Influenza in Health Care Settings for more information.
- Influenza testing may help inform decisions on infection prevention and control practices. See Prevention Strategies for Seasonal Influenza in Health Care Settings for more information.
- Persons who are at Higher Risk of Complications from Influenza include those aged ≥65 years or <2 years; pregnant women; persons with chronic lung disease (including asthma), heart disease, renal, metabolic, hematologic and neurologic disease; immunosuppression; and morbid obesity; American Indians or Alaska Natives, and residents of chronic care facilities.
- Antiviral treatment is recommended as soon as possible for outpatients with suspected or confirmed influenza who are at high-risk for complications from influenza, or those with progressive disease not requiring hospital admission. Outpatients who are not at higher risk of complications from influenza can be considered based upon clinical judgement if presenting within 2 days of illness onset. Guidance on antiviral treatment of influenza is available.
RIDTs
Rapid influenza diagnostic tests (RIDTs) are the most common flu tests (for influenza with moderate sensitivity (50%–70%) and high specificity). These tests do not provide information on influenza A subtypes. Nasopharyngeal, or nasal aspirates, swabs, or washes are all types of ways to provide specimens for RIDTs. Results can be ready in under 20 minutes; however, RIDTs may not be the most accurate of testing methods as they test antigens, so these tests can provide patients with a false negative result.
“Tests with low to moderate sensitivity and high specificity can produce false negative results more commonly than false positive results, especially during peak influenza activity in the community. Because of the lower sensitivity of the rapid influenza diagnostic tests, clinicians should consider confirming negative test results with molecular assays, especially during periods of peak community influenza activity and/or during suspected institutional influenza outbreaks because of the possibility of false-negative RIDT results. In contrast, false-positive RIDT results are less likely, but can occur and are more common during periods of low influenza activity.”3
Molecular assays
According to the CDC, rapid molecular assays (another type of flu test that can detect influenza virus nucleic acids in upper respiratory tract specimens with high sensitivity (90%–95%) and high specificity) are more accurate than RIDTs, can identify the presence of the genetic material of the flu virus, and have results ready in 20 minutes or less. Some rapid molecular assays are CLIA-waived for point-of-care use.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and other molecular assays can identify the presence of influenza viral RNA or nucleic acids in respiratory specimens with very high sensitivity and specificity. Some molecular assays are able to detect and discriminate between infections with influenza A and B viruses; other tests can identify specific seasonal influenza A virus subtypes. These assays can yield results in approximately 45 minutes to several hours depending upon the assay.3
Sensitivities and specificities of RT-PCR and other molecular assays that have been cleared by the FDA for diagnostic use are very high compared to other FDA-cleared assays that use different methods. However, even with RT-PCR, false negative results can occur due to improper or poor clinical specimen collection or from poor handling of a specimen after collection and before testing. A negative result can also occur by testing a specimen that was collected when the patient is no longer shedding detectable influenza virus.4
Immunofluorescence assays
Another assay to detect influenza A and B are immunofluorescence assays. These assays are antigen detection assays that generally require a fluorescent microscope to produce results in approximately 2–4 hours with moderate sensitivity and high specificity. Both direct fluorescent antibody and indirect fluorescent antibody staining assays detect influenza A and B viral antigens in respiratory tract specimens. Subtyping or further identification of influenza A viruses is not possible by immunofluorescence assays. One rapid immunofluorescence assay utilizes an analyzer device to produce results in approximately 15 minutes.3
Serologic testing and viral culture
The CDC does not recommend serologic testing for clinical decision making because the testing results for antibodies to influenza A or B viruses on a single serum specimen cannot be reliably interpreted. “Proper serological testing for diagnosis of influenza requires paired acute and convalescent sera collected 2–3 weeks apart, with reliable testing at a limited number of public health or research laboratories to assess a fourfold or greater rise in influenza virus strain-specific antibodies. Therefore, serological testing for influenza does not provide timely results to help with clinical decisions-making and is not recommended except for research and public health investigations.”3
Viral culture does not produce results in time for clinical decision making; a traditional tissue-cell culture can take up to 10 days. However, respiratory samples for viral culture are crucial for characterization of new seasonal influenza A and B virus strains.
Influenza SARS-CoV-2 (Flu SC2) multiplex assay
This test is an RT-PCR test that detects and differentiates RNA from SARS-CoV-2, influenza A virus, and influenza B virus in upper or lower respiratory specimens. The assay provides a sensitive, nucleic-acid-based diagnostic tool for evaluation of specimens from patients in the acute phase of infection.5 The FDA granted emergency use authorization (EUA) for this test on July 2, 2020. Because this is a multiplex assay, laboratories can process more tests in a given time period, using less reagents.
For further information on influenza testing methods, see Figure 2.6
Information for laboratorians
There are many factors that can affect test results — for that reason, care when selecting tests and following the recommended testing procedures are important.
When selecting a test, laboratorians should inform themselves on the tests they are considering by reading published studies on the tests or contacting the manufacturers. “Tests that have high sensitivity and high specificity will provide higher positive and negative predictive values, respectively. Tests that are CLIA-waived can be used at the point-of-care; tests that are classified as moderately complex are required to be performed in a clinical laboratory,” according to CDC guidance on influenza testing.7
Not following suggested procedures can result in false positive or false negative results. Alterations that affect results can include, “Using specimens for which the test is not optimized, using swabs that did not come with the rapid influenza diagnostic test kits (unless recommended), and improper storage or prolonged storage before specimens are tested.”7
The CDC’s “Influenza Specimen Collection” provides specific guidance on the materials needed and procedures to follow for testing using a nasopharyngeal swab, nasopharyngeal/nasal aspirate, nasopharyngeal/nasal wash, deep nasal swab, and a combined nasal and throat swab.8 Materials needed, depending on the specimen, include sterile swabs or sterile suction apparatuses and viral transport media tubes. Storing, packing, and shipping guidelines for specimens are as follows:
- Specimens should be placed into sterile viral transport media and immediately placed on refrigerant gel packs or at 4 degrees Celsius (refrigerator) for transport to the state public health laboratory.
- Keep specimens refrigerated (2–8 degrees Celsius, 26–46 degrees Fahrenheit) prior to shipping.
- Label specimen’s viral transport media tube with permanent marker or bar code. The CDC recommends not using a pencil or pen as they can smear or rub off.
- Ensure the cap on the tube is sealed tightly.
- Include a frozen cold pack with specimens.
- Ship specimens for testing as soon as possible.
- If delivery will be delayed for more than 3–4 days, specimen should be frozen at -70 degrees Celsius (-94 degrees Fahrenheit).
- Ensure specimen will be received by the public health laboratory during normal business hours.
- Additional considerations for influenza specimen collection:
- A nasopharyngeal (NP) swab is the optimal upper respiratory tract specimen collection method for influenza testing. However, such specimens cannot be collected from infants and many older patients may not allow an NP specimen to be collected. Alternatively, a combined nasal and throat swab specimen or aspirate specimens can provide good influenza virus yield.
- Some influenza tests are approved only for use with certain kinds of respiratory tract specimens, so follow guidelines provided by test. Also, some tests (e.g., rapid influenza diagnostic tests) are only approved for certain kinds of respiratory tract specimens.
- For best results (i.e., highest influenza virus yield), collect respiratory tract specimens within four days of illness onset.
- Most sensitive and accurate tests for influenza virus detection are molecular or nucleic acid amplification tests (RT-PCR).
- Negative test results obtained from rapid influenza diagnostic tests (RIDTs) that detect influenza viral antigens do not exclude influenza virus infection in patients with signs and symptoms of influenza. A negative test result could be a false negative and should not preclude further diagnostic testing (such as RT-PCR) and starting empiric antiviral treatment.
Conclusion
There are a number of tests that can help in the diagnosis of influenza. The most common are called “rapid influenza diagnostic tests” (RIDTs). RIDTs work by detecting the parts of the virus (antigens) that stimulate an immune response. These tests can provide results in less than 20 minutes but may not be as accurate as other flu tests. The rapid molecular assays detect genetic material of the flu virus. Rapid molecular assays can also produce results in less than 20 minutes and are more accurate than RIDTs. In addition to RIDTs and rapid molecular assays, there are several more accurate flu tests available that must be performed in specialized laboratories, including RT-PCR, viral culture, and immunofluorescence assays. With a possibly more severe flu season approaching, it is important for labs to review testing methods and prevention techniques to ensure that they are prepared.
References
- Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68(6):e1-e47. doi:10.1093/cid/ciy866.
- Guide for considering influenza testing when influenza viruses are circulating in the community. CDC.gov. Published May 6, 2021. Accessed July 28, 2022. https://www.cdc.gov/flu/professionals/diagnosis/consider-influenza-testing.htm.
- Overview of influenza testing methods. CDC.gov. Published May 6, 2021. Accessed July 28, 2022. https://www.cdc.gov/flu/professionals/diagnosis/overview-testing-methods.htm.
- Information on rapid molecular assays, RT-PCR, and other Molecular Assays for Diagnosis of Influenza Virus Infection. CDC.gov. Published October 21, 2019. Accessed July 28, 2022. https://www.cdc.gov/flu/professionals/diagnosis/molecular-assays.htm.
- CDC’s influenza SARS-CoV-2 multiplex assay and required supplies. Centers for Disease Control and Prevention. Published October 15, 2021. Accessed July 29, 2022. https://www.cdc.gov/coronavirus/2019-ncov/lab/multiplex.html.
- Influenza virus testing methods. CDC.gov. Published August 10, 2020. Accessed July 28, 2022. https://www.cdc.gov/flu/professionals/diagnosis/table-testing-methods.htm.
- Rapid Diagnostic Testing for Influenza: Information for Clinical Laboratory Directors. Cdc.gov. Published February 4, 2019. Accessed July 28, 2022. https://www.cdc.gov/flu/professionals/diagnosis/rapidlab.htm#table3.
- Influenza Specimen Collection. CDC.gov. Published July 2, 2020. Accessed July 28, 2022. https://www.cdc.gov/flu/pdf/freeresources/healthcare/flu-specimen-collection-guide.pdf.