Once the novel coronavirus (SARS-CoV-2) hit the United States, Central Ohio Primary Care, with more than 75 locations, wanted to launch polymerase chain reaction (PCR) testing to detect the virus.
And it did – eventually.
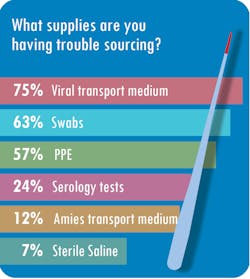
But not without a protracted effort to overcome stumbling blocks. “We’ve had a lot of trouble getting test reagents,” said Cynthia Roberts, MBA, MLT (ASCP), Laboratory Manager at Central Ohio Primary Care, based in Westerville. The lab also has struggled to source nasopharyngeal swabs, she said.
Central Ohio Primary Care – which performs four million tests annually and operates three patient draw centers – already had the Cepheid GeneXpert in-house, but it also purchased the DiaSorin Molecular Liaison MDX and the Abbott ID Now, Roberts said. With a combination of the three platforms, the lab ramped up its capacity to 90 tests per day.
The healthcare provider is not alone. Many of Roberts’ peers at other laboratories described similar issues in Medical Laboratory Observer’s (MLO) third State of the Industry survey, which focuses on disease management and testing for COVID-19.
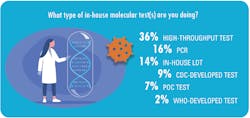
Most of the labs with in-house PCR testing to detect SARS-CoV-2 (63 percent) are conducting a modest number of tests per day, or between one and 50 tests.
Among the other labs that responded to MLO’s survey, the breakdown of daily test volume is as follows:
• 51-200 tests for 18 percent
• 201-500 for 10 percent
• 501-1,000 for 6 percent
• 1,001-and 2,000 for 2 percent
• More than 2,000 tests for 1 percent
The majority, or 72 percent, of survey respondents work at hospital labs, while 12 percent work in physician’s office labs, 7 percent work in integrated clinical labs, 3 percent work in government labs, 2 percent work in independent labs, and 1 percent work in reference labs. Three percent of survey respondents selected the “other” category when asked what type of lab they work in.Sourcing Supplies
Nearly half of respondents, or 49 percent, said sourcing supplies has been the single biggest challenge during the pandemic. Other respondents noted other challenges, such as communicating effectively (16 percent), sourcing PPE (14 percent), scaling up to the volume of testing necessary (13 percent) and scheduling personnel (8 percent).Roberts said Central Ohio Primary Care has had trouble getting vendors’ attention. “Companies are prioritizing who gets supplies, and we weren’t made a priority. Then it took weeks to months to be able to purchase new platforms because supply couldn’t meet demand,” Roberts said. “Many things are allocated based on prior usage – if you weren’t using NP (nasopharyngeal) swabs or VTM (viral transport media) prior to this crisis, they wouldn’t sell you any,” she said.
SwedishAmerican Hospital in Rockford, IL, collects about 120 specimens per day but sends out more than 100 of those to reference labs. The hospital, a division of University of Wisconsin Health, simply has not been able to source supplies or new testing platforms, according to Nicole Radford, FACHE, MS, MT(ASCP), laboratory director at SwedishAmerican.
Finding enough testing capacity at reference labs also has been difficult, she said.
“For the various reference labs that we’ve used, we’ve noted that, even if they don’t discuss it, there are only so many that they can do within the established turnaround time. Some of the labs we’ve used have accepted anything sent their way, but the turnaround time increases to (at times) unreasonable levels. Other labs will have put a cap on the amount that will be accepted per client in order to protect the turnaround time. Either way, this has presented a problem for our lab’s offering of COVID-19 testing,” she said.
As a result, she said SwedishAmerican has switched reference labs a number of times. While this led the lab managers to rework their process multiple times, it also has allowed the hospital to meet the demand for testing.
Finding new analyzers during the pandemic, which would allow SwedishAmerican to perform more tests in-house, also has not been easy. They are “very few and far between at this point,” Radford said. The hospital inked a contract for a high-throughput machine before the pandemic but still has not taken delivery of it. “I’ve heard time and again from a number of vendors that precedence and priority are given to clients who are obtaining the equipment with capital funds (outright purchase) versus leasing or reagent rental options,” Radford said.
For their part, vendors have also had to react to the havoc caused by the pandemic.
Timothy Templet, executive vice president of sales at Puritan Medical Products, Guilford, ME, said, “During these unprecedented times, Puritan is managing its current manufacturing capabilities while working diligently to increase capacities over three product categories with additional manufacturing locations all based in the USA. Puritan continues to receive multiple requests for COVID-19 testing swabs from new potential customers and we are doing our best to accommodate everyone now and in the future.”
The company makes foam, spun polyester and flock swabs and primarily sells them to vendors of testing platforms and distributors.
Supply chain solutions
Because of the shortages of supplies, such as swabs, many labs are conducting SARS-CoV-2 tests on multiple vendors’ platforms. “We encourage our clients to develop two, three or even four different platforms for testing. That’s our short-term solution,” said David Nichols, President and Founder of Nichols Management Group, which specializes in consulting services to laboratories.Nichols said he thinks supply shortages, particularly for PPE, have leveled out somewhat since MLO conducted its survey in April and May. “I have spoken to a number of health systems in the last couple of days who have said they are 100 percent back in inventory for their PPE. It has really eased up,” Nichols said. However, he also added that “it is hard to generalize for the country. It is back to inventory levels, but there still are pockets of need.”
He urges clients to move from a short-term crisis response to medium- and long-term planning, so they will be prepared when subsequent waves of COVID-19 potentially crash through their doors. He suggests labs evaluate their current automation platforms and lease commitments, with a goal of lowering their cost-per-test. “We believe that the coronavirus will come and go in peaks and valleys, and there will be COVID-19 related testing for an extended period of time,” he said.
“I would urge organizations to migrate to the lowest cost platform that is appropriate and secure the supplies. If you are working diligently on it now, it is possible to get them in place by late fall,” he said.
Taking a long-term approach to purchase anlyzers is the route Radford from SwedishAmerican has followed since the beginning. She said, “We have to consider the future post-COVID. Will this be a platform that we’ll be ‘stuck’ with later? Will we be able to use the other tests on the menu when and if the number of COVID test requests are no longer the number one ordered test?”
“There’s an example of a vendor whose equipment I strongly considered. While the analyzer would certainly be helpful for COVID testing, it would not be needed later because we already have more cost-effective and reliable methods of performing all of the other tests on the instrument’s menu,” she explained.
Reimbursement woes
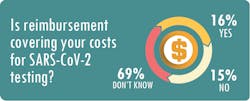
Nichols said his clients are spending between $40 and $150 per test, compared to reimbursement rates from the Centers for Medicare & Medicaid Services (CMS) of about $51 for a standard PCR assay and $100 for a high-throughput test.
As Roberts notes, that margin hardly covers test kits – not to mention overhead such as rent, salaries and PPE.
At SwedishAmerican, the expenses for the in-house tests are not “far below” the Medicare reimbursement rate of $100 per test, and the cost of each test sent to outside labs “is very close to or more than $100,” Radford said.Nichols recommends that labs form a committee comprised of employees from operations, billing and information technology to make sure they are capturing the appropriate revenue for the testing services. This involves such steps as capturing accurate demographic information, utilizing the proper billing codes and closely tracking payments from government and commercial payers.
“This needs to be a robust effort because guidelines are changing and practices are changing. If that is not done, the lab will find that COVID-19 testing will lead to a very significant reduction in their operating margin,” he said.
MLO also asked survey respondents if the revenue generated from SARS-CoV-2 tests plus other tests performed on patients with a confirmed diagnosis of COVID-19 was enough to offset the reduced test volumes associated with elective procedures. A total of 58 percent said “no,” 4 percent said “yes,” and 38 percent said the question did not apply to their situation.
When asked what other types of tests clinicians are ordering for patients with confirmed or suspected cases of COVID-19, 44 percent said D-dimer value, 35 percent said cardiac enzymes, 28 percent said glucose, 8 percent said ferritin, and 28 percent selected the “other” category. Slightly more than one-third, or 39 percent, said they are not processing other tests for COVID-19 patients.
POC testing
In addition to lab-based testing, Central Ohio Primary Care also added POC testing with the Abbott ID Now system, which uses dry swabs. However, Roberts said she has assigned a laboratory staff member to oversee the testing process at the patient draw center, ensuring the appropriate procedures are followed and test results are accurate.
Providers are collecting specimens at a variety of venues, according to survey respondents, including emergency departments (72 percent of respondents), drive-up testing (56 percent of respondents), physician’s offices (40 percent of respondents) and urgent care centers (39 percent of respondents).Workflows and training
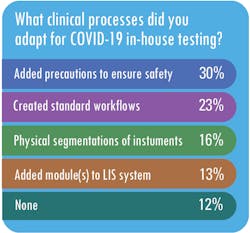
MLO also asked respondents what changes they made to their work processes to make in-house testing for SARS-CoV-2 possible.
While a majority of respondents, said they did not have to modify their existing guidelines for infectious disease testing to accommodate testing for SARS-CoV-2, Radford said SwedishAmerican has made some modifications to its infectious-disease processes for COVID-19.For example, she said, “we are testing all patients within 72 hours prior to a planned procedure that fit specific criteria of risk. While this is not unlike other surveillance that we may do (i.e. MRSA screening), what is different is that the patient must self-isolate for the 72 hours prior to the procedure,” Radford said.
For labs using the high-throughput testing apparatus, they used a variety of approaches to train staff on the machines. Nearly two-thirds, or 62 percent, used a train-the-trainer approach, while 31 percent used training provided by the vendors, 13 percent used online training modules, and 7 percent used lunch-and-learn sessions.
Serology testing
In addition to molecular testing to diagnose active cases of COVID-19, some providers have added serology testing to detect the antibodies the body produces to fight off viruses such as SARS-CoV-2, known as immunoglobulin M (IgM) and immunoglobulin G (IgG).
Indeed, Central Ohio Primary Care launched serology testing in May. But the Ohio provider is in the minority. According to the MLO survey, less than a third of survey respondents said they have added serology testing.
Nichols thinks that all labs certified to provide moderate complexity testing and above should add serology testing. It is not only a valuable service in high demand but also yields an acceptable margin benefiting lab budgets.
“The good news is it seems that the reimbursement for the antibody testing, which is largely going to be performed on large-scale automated equipment, has a significantly higher margin than the antigen testing,” Nichols said. “I would think every one of the top vendors will have this serology capability on an automated platform within three months,” he said.
After all, he notes, “the demand for this kind of testing may not be going to go away.”