Human immunodeficiency virus (HIV) is an RNA (ribonucleic acid) virus that infects human immune cells, in particular CD4 T lymphocytes and is acquired through mucosal or intravenous exposure to infected bodily fluids such as blood, semen, and genital tract secretions. Infection from HIV was first reported in 1981 in the Morbidity and Mortality Weekly Report (MMWR), a publication of the Centers for Disease Control and Prevention (CDC), when doctors discovered clusters of Kaposi's sarcoma and pneumocystis pneumonia in homosexual men in Los Angeles, New York City, and San Francisco having weakened immune systems. In 1982, the CDC named the disease caused by HIV as acquired immune deficiency syndrome, AIDS.1 If left untreated, HIV infections progress from acute through chronic to AIDS. Excepting a small proportion of untreated persons infected with HIV who remain asymptomatic and show little evidence of progressive immune suppression after 10 or more years of infection, nearly all eventually develop AIDS. The number of AIDS cases grew in epidemic proportion and per the latest United Nations AIDS factsheet, 85.6 million people have become infected with HIV, and 40.4 million people have died from AIDS-related illnesses since the start of the epidemic.2
HIV has two subtypes – HIV-1 and HIV-2. HIV-1 has 3 major phylogenetic groups – M (major), O (outlier) & N (non-M, non-O), all of which can cause AIDS.3,4,5 HIV-1 M infections are common worldwide, O is restricted to West Central Africa and N is least spreading and was found in Cameroon.3 HIV-1 group N is a mosaic of divergent HIV-1 and simian immunodeficiency virus (SIV) found in chimpanzees.4,5 The HIV strains reported in the United States in 1981 were of HIV-1 subtype. Serologic evidence of another human immunodeficiency virus subtype (HIV-2) was discovered in 1985 in Senegal,6 which was subsequently isolated in 1986 from a Cape Verdean patient.7 Around 95% of the people infected with HIV are infected with HIV-1 subtype. HIV-2 is mainly present in West Africa, but it is slowly starting to appear in other regions, including the United States, Europe, and India.8
Situations that necessitate testing
HIV testing is needed as follows:
- To determine the human immunodeficiency virus (HIV) infection status of a person
- To test or screen blood products before donation
- To evaluate the progression of disease
- To monitor the effectiveness of antiretroviral therapy (ART)
Tests for HIV detection
HIV infection can be detected in the following ways: 9
- Direct visualization of HIV virus by electron microscopy
- Cultivation by lymphocyte culture
- Measurement of HIV-specific serologic responses
- Detection of viral antigens
- Detection of viral nucleic acids
However, the most common tests use the immunological method or the molecular method.10
Enzyme-linked immunosorbent assays (ELISA)
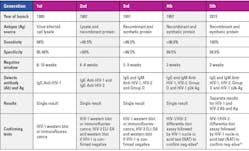
The first-generation HIV test was an immunological test that became available in 1985 — the enzyme-linked immunosorbent assay (ELISA) detected IgG antibody of HIV-1 only in patients 6 to 12 weeks after infection. False-positive results occurred; so positive results needed to be confirmed using a second test — western blot or an immunofluorescence test. The second-generation HIV test became available in 1987 and had increased specificity and positive predictive value as it had a recombinant HIV-1 p24 antigen. Some tests included HIV-1 group O protein and an HIV-2 protein to enable detection of both HIV-1 and HIV-2 antibodies. The second-generation tests also reduced the antibody-negative window to 4 to 6 weeks post-infection infection. The third-generation HIV tests added an IgM antibody and reduced the antibody-negative window further to three weeks post-infection. In the late 1990s, the fourth-generation HIV screening assays were launched that detected both antibody and p24 antigen for HIV-1 and HIV-2 combined and reduced the test-negative to two weeks. In 2015, the fifth-generation HIV screening assay was launched that not only detected both antibody and antigen for HIV-1 and HIV-2 but also differentiated between HIV-1 and HIV-2.11 Table 1 summarizes the evolution of HIV detection assays.
Molecular method/nucleic acid test (NAT)
Nucleic acid tests can be used as follows:
- To diagnose HIV patients
- To determine disease progression and enable antiretroviral treatment monitoring
- Determine drug resistance characterization
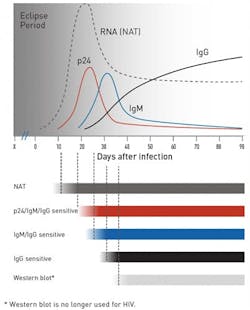
A nucleic acid test for HIV-1 was first demonstrated in blood specimens from HIV-1 infected individuals in 1988.13 It detects the HIV RNA and has the shortest window period of 10 to 33 days post-infection. NAT is considered more accurate than ELISA tests and hence used as confirmatory tests. However, NATs are more expensive than ELISA tests. The graph in Figure 1 depicts the window period for the different types of tests — the NAT detects HIV the earliest post-infection, followed by the antigen/antibody test, and lastly, the antibody test.14
HIV Screening and testing guidelines
In the United States, screening of blood donors for HIV-1 and HIV-2 started in March 1985 and June 1992 respectively.15
At present, the CDC recommends that everyone between the ages of 13 and 64 should get tested for HIV at least once as part of their routine health care. Those with certain ongoing risk factors — such as having more than one sex partner since their last HIV test or having sex with someone whose sexual history they don’t know — should get tested annually. Some sexually active gay and bisexual men may benefit from more frequent testing (e.g., every three to six months).16
Specimen types for HIV testing include blood from a finger stick or in the form of serum, plasma, or dried blood spots (DBS). Oral fluid and urine specimens can also be used for HIV testing.17
Disease progression and evaluation
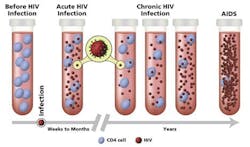
There are three stages of HIV infection, including acute, chronic, and AIDS.18
Acute HIV infection: Develops within two to four weeks after infection. Some people get flu-like symptoms, such as fever, headache, and rash. HIV multiplies rapidly and spreads throughout the body. The virus attacks and destroys the CD4 T lymphocytes. As the level of HIV in the blood is very high, the risk of transmission is also very high during this stage.
Chronic HIV infection: Is the next stage when HIV continues to multiply in the body but at very low levels and hence the individuals are mostly asymptomatic. They can transmit HIV to others but if the viral load is maintained at an undetectable level taking antiretroviral therapy (ART), risk of HIV transmission is minimal.
Acquired immune deficiency syndrome (AIDS): The most severe stage when the individual’s immune system is damaged and cannot fight off infections from opportunistic organisms.
There are two types of tests to evaluate disease progression: HIV viral load and CD4 count. HIV viral load tests are quantitative nucleic acid tests that determine the number of copies of HIV RNA per milliliter of blood. They can be used to diagnose acute HIV infection, guide treatment choices, and monitor response to antiretroviral therapy (ART).19
CD4 count determination by immunofluorescence analysis using flow cytometer is considered the gold standard. The normal range is between 500 to 1500 cells/cubic millimeter. For AIDS patients CD4 cell count drops below 200 cells/cubic millimeter.20 Figure 2 depicts the HIV viral load and CD4 count during HIV progression.
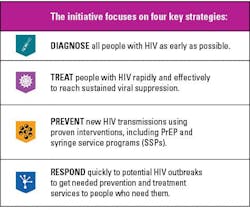
Ending the HIV Epidemic Initiative
With various developments in HIV testing and treatment options, the HIV epidemic has seen a decline with new infections declining. In 2019, the U.S. Department of Health and Human Services (HHS) launched an initiative that aims to reduce new HIV infections in the United States by 90 percent by 2030 (See Figure 3).23
However, the emergence of drug resistant HIV strains has posed a great challenge to successful antiretroviral therapy (ART).21 The HIV-1 pol gene is generally used to identify mutations associated with drug resistance and also for HIV-1 subtyping.22
Conclusion
It is often said that “Where there is a will, there is a way.” Tackling the HIV epidemic proves that point. The menace from the devastating HIV epidemic is now under control, thanks to the efforts by the various sections of society. Now, HIV testing is not restricted to clinical labs and is available to the public as point-of-care at-home tests. Hence by following the tenets of EHE, one can prevent infection from HIV, and if by chance get infected, individuals should get diagnosed and treated immediately to prevent development into AIDS.
References
1. Gov H. Timeline of the HIV and AIDS epidemic. Hiv.gov. Published November 15, 2023. Accessed December 14, 2023. https://www.hiv.gov/hiv-basics/overview/history/hiv-and-aids-timeline/.
2. Global HIV & AIDS statistics — Fact sheet. Unaids.org. Accessed December 14, 2023. https://www.unaids.org/en/resources/fact-sheet.
3. Yang C, Dash B, Hanna SL, et al. Predominance of HIV type 1 subtype G among commercial sex workers from Kinshasa, Democratic Republic of Congo. AIDS Res Hum Retroviruses. 2001;1;17(4):361-5. doi:10.1089/08892220150503726.
4. Yang C, Dash BC, Simon F, et al. Detection of diverse variants of human immunodeficiency virus-1 groups M, N, and O and simian immunodeficiency viruses from chimpanzees by using generic pol and env primer pairs. J Infect Dis. 2000;181(5):1791-5. doi:10.1086/315439.
5. Fonjungo PN, Dash BC, Mpoudi EN, et al. Molecular screening for HIV-1 group N and simian immunodeficiency virus cpz-like virus infections in Cameroon. AIDS. 2000;14;14(6):750-2. doi:10.1097/00002030-200004140-00018.
6. Barin F, M'Boup S, Denis F, et al. Serological evidence for virus related to simian T-lymphotropic retrovirus III in residents of west Africa. Lancet. 1985;21-28;2(8469-70):1387-9. doi:10.1016/s0140-6736(85)92556-5.
7. Clavel F, Guétard D, Brun-Vézinet F, et al. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986;18;233(4761):343-6. doi:10.1126/science.2425430.
8. Burgess L. HIV-1 vs. HIV-2: Differences and similarities. Medicalnewstoday.com. Published December 5, 2018. Accessed December 14, 2023. https://www.medicalnewstoday.com/articles/323893.
9. Tang YW, Ou CY. Past, present and future molecular diagnosis and characterization of human immunodeficiency virus infections. Emerg Microbes Infect. 2012;1(8):e19. doi:10.1038/emi.2012.15.
10. HIV testing. Cdc.gov. Published June 9, 2022. Accessed December 14, 2023. https://www.cdc.gov/hiv/testing/.
11. Alexander TS. Human Immunodeficiency Virus Diagnostic Testing: 30 Years of Evolution. Clin Vaccine Immunol. 2016;4;23(4):249-53. doi:10.1128/CVI.00053-16.
12. 2018 Quick reference guide: Recommended laboratory HIV testing algorithm for serum or plasma specimens. Published online 2018. Accessed December 14, 2023. https://stacks.cdc.gov/view/cdc/50872.
13. Ou CY, Kwok S, Mitchell SW, et al. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988;15;239(4837):295-7. doi:10.1126/science.3336784.
14. Which HIV tests should I use? Cdc.gov. Published June 1, 2023. Accessed December 14, 2023. https://www.cdc.gov/hiv/clinicians/screening/tests.html.
15. U.S. public health service guidelines for testing and counseling blood and plasma donors for human immunodeficiency virus type 1 antigen. Cdc.gov. Published March 1, 1996. Accessed December 14, 2023. https://www.cdc.gov/mmwr/preview/mmwrhtml/00040546.htm.
16. Gov HIV. Resources for 2023 national HIV Testing Day. Hiv.gov. Published June 15, 2023. Accessed December 14, 2023. https://www.hiv.gov/blog/resources-for-2023-national-hiv-testing-day/.
17. Women and HIV: Get the Facts on HIV Testing, Prevention, and treatment. U.S. Food and Drug Administration. Published October 30, 2023. Accessed December 14, 2023. https://www.fda.gov/consumers/womens-health-topics/women-and-hiv-get-facts-hiv-testing-prevention-and-treatment.
18. The stages of HIV infection. Nih.gov. Accessed December 14, 2023. https://hivinfo.nih.gov/understanding-hiv/fact-sheets/stages-hiv-infection.
19. Viral load test. Hiv.gov. Accessed December 14, 2023. https://clinicalinfo.hiv.gov/en/glossary/viral-load-test.
20. Battistini Garcia SA, Guzman N. Acquired Immune Deficiency Syndrome CD4+ Count. StatPearls Publishing; 2023.
21. Chimukangara B, Samuel R, Naidoo K, de Oliveira T. Primary HIV-1 Drug Resistant Minority Variants. AIDS Rev. 2017;19(2):89-96.
22. Pasquier C, Millot N, Njouom R, et al. HIV-1 subtyping using phylogenetic analysis of pol gene sequences. J Virol Methods. 2001;94(1-2):45-54. doi:10.1016/s0166-0934(01)00272-5.
23. Introduction. Cdc.gov. Published August 9, 2021. Accessed December 14, 2023. https://www.cdc.gov/hiv/policies/strategic-priorities/mobilizing/introduction.html.