Biopsy, generally referred to tissue biopsy, is a procedure where a tissue or a cell is taken from the body and tested for cancer. It uses a morphology-based technique to examine cancer cells. Based on the anatomical site, the specimen is collected using a needle; an image-guided procedure such as CT scan, MRI, or ultrasound; an endoscopic procedure; or surgical procedure.
Tissue biopsy versus liquid biopsy
Limitations of tissue biopsies:1,2
- Tissue biopsies are an invasive method and difficult to collect for some anatomical sites.
- They provide very limited information on the intratumoral and intermetastatic genetic heterogeneity and the genetic and epigenetic changes that occur with disease progression. Thus, the therapeutic decisions must be based on historical tissue biopsy results, which may be suboptimal.2
- They cannot identify any lesions in different locations.
- Surgical biopsies:
- Cannot be repeated or done frequently
- Dependent on patient’s age and health condition
- Involve high cost
- Can cause harmful clinical complications.2 Depending on the tissue biopsy procedure, patients may experience excessive bleeding (hemorrhage), infection puncture damage to nearby tissue or organs, and skin numbness around the biopsy site.3
On the other hand, liquid biopsy, which is based on the molecular profiling of the biofluids, is able to overcome some of the above-mentioned limitations posed by tissue biopsy.1
Advantages of liquid biopsy tests:
- Liquid biopsy is minimally invasive and hence does not pose the challenges to the patient that invasive surgical tissue biopsies may cause.
- Specimens for liquid biopsy are body fluids and can be collected frequently.
- Analysis of liquid biopsy tests can provide various information in real-time including mutations in DNA, copy number alterations (CNA)s of crucial genes, transcriptome/proteome profiling, epigenetic alterations, and metabolite profiling, which would provide crucial longitudinal information and data for more accurate diagnosis by the pathologists regarding both primary and metastasized tumors.4 This information would help to understand the state of the tumor, progressive genetic and epigenetic changes occurring in the tumor, and multiple metastatic alterations, allowing proper therapeutic management of the cancer patient.2
Background on liquid biopsy
In 1869, Thomas Ashworth discovered “some cells” in the blood of a deceased patient with metastatic cancer that looked like the primary tumor cells.5 They were later called circulating tumor cells (CTCs). CTCs are tumor cells that have detached from the primary tumor or metastatic site and got swept into the circulatory or lymphatic system.6 Catherine Alix-Panabières and Klaus Pantel coined the term “liquid biopsy” in 2010 when studying circulating tumor cells (CTCs) in cancer patients’ blood.7
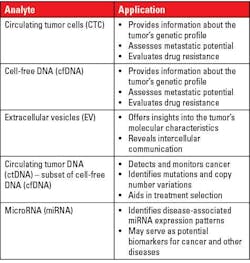
Analytes for liquid biopsy tests:
Currently liquid biopsy (LB) is not restricted to CTCs only and has extended to other biomolecules. The various analytes for liquid biopsy can be classified into two main categories:1
- Large or small molecules without cells or without a subcellular structure in the body fluid such as proteins, nucleic acids (e.g., cell-free DNA [cfDNA], cell-free RNA [cfRNA], microRNA), lipids, carbohydrates, and other small metabolites and metal ions
- Cellular or subcellular structures such as single or clustered circulating tumor cells (CTCs), circulating cancer-related fibroblasts (CAF), immune cells, tumor-educated platelets (TEP), exosomes or extracellular vesicles (EVs), and circulating mitochondria
In addition to blood, liquid biopsies have used other body fluids such as urine, saliva, cerebrospinal fluid (CSF), pleural effusion, bone marrow, sputum, etc.8,9
Tissue biopsy is still considered the gold standard for cancer diagnosis and prognosis. That said, liquid biopsy is rapidly evolving and has already shown great promise in diagnosis and treatment of cancer patients.
Potential applications of liquid biopsy:9,10
- Early detection of cancer using high blood volumes
- Identification of minimal residue disease - a small number of cancer cells left in the body after treatment. These cells have the potential to come back and cause relapse.
- High-risk population screening
- Determining stage of cancer and disease recurrence monitoring
- Monitoring treatment response
- Identifying resistance mechanism
Table 1 lists the possible applications based on the type of analyte.9
FDA-approved liquid biopsy tests
Though studies on liquid biopsies have shown promise for various applications, the liquid biopsy assays approved so far by the U.S. Food and Drug Administration (FDA) are used to identify eligibility for certain targeted treatments, gauge the response to therapy, and monitor disease progression in patients with lung, breast, prostate, colorectal, ovarian, and other solid cancers.10
Similarly, even though studies have been conducted using various analytes for the liquid biopsy tests, the liquid biopsy tests that have been approved are based on circulating tumor cells (CTCs), and circulating tumor DNA (ctDNA).10
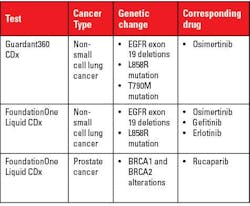
The list of FDA-approved liquid biopsy tests are as follows:11
- Cell Search® Circulating Tumor Cell (CTC) Test detects CTCs. It is used to predict the likely outcome for people with metastatic breast, prostate, or colon cancer.
- Cobas® EGFR Mutation Test v2 detects ctDNA. It detects a mutation on the EGFR gene in non-small cell lung cancer (NSCLC).
- Guardant360® CDx detects ctDNA.
- FoundationOne® Liquid CDx detects ctDNA.
Guardant360® CDx and FoundationOne® Liquid are approved for companion diagnostics and general tumor profiling. Table 2 provides details.12
The most common technological methods that have been used for liquid biopsy are as follows:13
- Traditional qPCR mutational testing
- Droplet digital PCR (ddPCR)
- Next-generation sequencing (NGS)
Newer technologies such as nanotechnology are being developed for liquid biopsy applications and have shown valuable promise.13,14
Limitations of liquid biopsy
Though liquid biopsies have great potential, they do have quite a few limitations. Some of the limitations are as follows:15
- Liquid biopsies are still not considered the primary test for cancer diagnosis and require an initial histologic diagnosis to be obtained by tissue biopsy, the gold standard.
- Assays for ctDNA do not provide any insight into many tumor properties and hence cannot be used to determine the stage and grade of cancer.
- Liquid biopsy is a new technique and hence biomarkers are not yet available for all types of cancers.
- The very low variant frequency in ctDNA can result in both false negatives if numbers are below an assay’s limit of detection and false positives due to background sequencing errors.
Conclusion
According to World Health Organization (WHO), cancer is the second leading cause of death worldwide, and in 2020, 10 million people died from cancer.16 Cancer is a diverse and complicated illness that is caused due to genetic and epigenetic changes in normal cells. By diagnosing cancer early and providing proper treatment, patients can be cured of cancer. Liquid biopsies are showing great promise as a diagnostic tool for early diagnosis of cancer, to monitoring cancer progression and guiding therapy. Moreover, with the integration of various technologies in genomics, proteomics, transcriptomics, and metabolomics that are taking place at a rapid pace in liquid biopsy research, it may not be too far off when liquid biopsies will be considered as the standard of care for cancer diagnosis and will aid in offering personalized therapy to cancer patients.
References
1. Armakolas A, Kotsari M, Koskinas J. Liquid Biopsies, Novel Approaches and Future Directions. Cancers (Basel). 2023;3;15(5):1579. doi:10.3390/cancers15051579.
2. Yu W, Hurley J, Roberts D, et al. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. 2021;32(4):466-477. doi:10.1016/j.annonc.2021.01.074. Biopsy. Gov.au. Accessed January 23, 2024. https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/biopsy.
3. Ashworth, T. R. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Australas. Med. J. 14, 146–149 (1869).
4. Lone, S.N., Nisar, S., Masoodi, T. et al. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol Cancer. 2022;18;21(1):79. doi:10.1186/s12943-022-01543-7.
5. Lin, D., Shen, L., Luo, M. et al. Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther. 2021;22;6(1):404. doi:10.1038/s41392-021-00817-8.
6. Pantel K, Alix-Panabières C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med. 2010;16(9):398-406. doi:10.1016/j.molmed.2010.07.001.
7. Fernández-Lázaro D, García Hernández JL, García AC, et al. Liquid Biopsy as Novel Tool in Precision Medicine: Origins, Properties, Identification and Clinical Perspective of Cancer's Biomarkers. Diagnostics (Basel). 2020;13;10(4):215. doi:10.3390/diagnostics10040215.
8. Alix-Panabières C, Marchetti D, Lang JE. Liquid biopsy: from concept to clinical application. Sci Rep. 2023;7;13(1):21685. doi:10.1038/s41598-023-48501-x.
9. Noor J, Chaudhry A, Noor R, Batool S. Advancements and Applications of Liquid Biopsies in Oncology: A Narrative Review. Cureus. 2023;31;15(7):e42731. doi:10.7759/cureus.42731.
10. Licciulli S. The expanding potential of liquid biopsy to detect and monitor cancer. American Association for Cancer Research (AACR). Published August 31, 2023. Accessed January 23, 2024. https://www.aacr.org/blog/2023/08/31/the-expanding-potential-of-liquid-biopsy-to-detect-and-monitor-cancer/.
11. Liquid biopsy. Cleveland Clinic. Accessed January 23, 2024. https://my.clevelandclinic.org/health/diagnostics/23992-liquid-biopsy.
12. FDA approves blood tests that can help guide cancer treatment. National Cancer Institute. Published October 15, 2020. Accessed January 23, 2024. https://www.cancer.gov/news-events/cancer-currents-blog/2020/fda-guardant-360-foundation-one-cancer-liquid-biopsy.
13. Alexandrou G, Mantikas KT, Allsopp R, et al. The Evolution of Affordable Technologies in Liquid Biopsy Diagnostics: The Key to Clinical Implementation. Cancers (Basel). 2023;16;15(22):5434. doi:10.3390/cancers15225434.
14. Goswami S, Samanta P, Adhikari MD. Nanobiotechnology: A smart platform of the future transform liquid biopsy era. The Journal of Liquid Biopsy. 2024;3(100137):100137. doi:10.1016/j.jlb.2024.100137.
15. The “liquid” biopsy. College of American Pathologists. Published March 22, 2022. Accessed January 23, 2024. https://www.cap.org/member-resources/articles/the-liquid-biopsy.
16. Cancer today. WHO International Agency for Research on Cancer. Estimated number of deaths in 2020, all cancers, both sexes, all ages. (2020). Accessed January 23, 2024. https://gco.iarc.fr/today/online-analysis-pie.