Walking up the stairs had become difficult for Louise, an otherwise healthy, active, and stubborn 85-year-old retired nurse. It was spring, and Louise figured that she was deconditioned and out of shape following a particularly snowy winter that kept her from her daily walks. But when she was struggling to breathe just sitting still and sweating on a cool spring day, her family insisted that she go to the emergency department. A short while later, doctors confirmed what she suspected. Louise had had a heart attack. Her left anterior descending artery (LAD), the artery feeding the left ventricle, was 95% blocked, which had caused significant cardiac damage. Louise, like millions of others around the world, now faces a new reality—congestive heart failure.
Statistics and trends
In 2021, medical organizations worldwide convened and proposed a global definition of heart failure “as a clinical syndrome with symptoms and/or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.”1 Simply, heart failure is characterized by inadequate cardiac output that occurs when the cardiac muscle fails to pump enough blood to keep up with the body’s needs. Symptoms of heart failure include dyspnea, fatigue, weakness, atrial fibrillation, and pulmonary and peripheral edema.
According to the World Heart Federation, individuals have a 20% lifetime risk of developing heart failure, one of the world’s leading causes of hospitalization.2 Worldwide, there are an estimated 26 to 64 million individuals with heart failure.3,4 And as the world’s population ages, the burden of heart failure is increasing.2,5 Of course, this heavy burden to human health comes with a financial strain. The global cost of heart failure is expected to rise from 30.7 billion USD in 2012 to 69.8 billion USD in 2030—nearly 40 billion USD in just 18 years.6
Even with proper disease management, heart failure has a high mortality rate, with a one-year mortality of up to 30% and a five-year mortality rate of up to 75%.6 These mortality rates are even higher in low and middle income countries.2
Disease causes and progression
Heart failure can occur on both the left and right sides of the heart—left-side heart failure is more common and, over time, can lead to right-side heart failure.7 Left-sided heart failure is classified into one of three groups based on left ventricular ejection fraction (EF): heart failure with reduced EF (HFrEF) when the EF is ≤40%, heart failure with mildly reduced EF (HFmrEF) when the EF is 41-49%, and heart failure with preserved EF (HFpEF) when the EF is ≥50%.6,8 (Normal ejection fraction is 55%–65%.) Generally, in HFpEF, the cardiac muscle doesn’t fully relax between beats, so it can’t fill up with enough blood to pump to the body, but the percentage of what is pumped remains high. Conversely, in HFrEF, the cardiac muscle is too weak to pump out enough blood.7 HFmrEF, a relatively new category of heart failure, is less understood but shares characteristics of both HFrEF and HFpEF.9,10 Understanding the type of heart failure is important to understanding disease etiology.11
There are a variety of causes of heart failure, with coronary artery disease and heart attack being among the most common. Heart failure can arise over time, as it did in Louise’s case. Reduced blood supply to her cardiac muscle led to cardiac weakening, which led to stretching and thickening of the cardiac chambers. When the heart grows in this way, it can’t pump efficiently. Conversely, heart failure can occur acutely as a consequence of heart attack where heart muscle dies. In these cases, there may not be enough remaining heart muscle left to properly pump the blood. Other causes include heart valve disease, cardiac infection (myocarditis, usually of viral origin), congenital heart defects, and high blood pressure.7 Aging, renal disease, unhealthy lifestyle choices such as poor diet, smoking and other drug use, and lack of physical exercise can also lead to weakening and stiffening of the heart.6,7,12
Although there is an approximately even distribution between men and women,13 heart failure may present differently between the sexes12 with an increased prevalence of HFpEF in females and an increased prevalence of heart failure with reduced ejection fraction HFrEF in males.12,14,15
Clinically, heart failure presents as a syndrome with typical signs and symptoms including shortness of breath (dyspnea), fluid retention in the extremities (edema), neurologic changes such as confusion, and exhaustion coupled with an underlying cardiac cause.11 Final diagnosis is based on symptoms, physical findings, electrocardiography and echocardiography, tests for ejection fraction, and blood tests for elevated levels of B-type natriuretic peptides (BNP and NT-proBNP).11 Regardless of the cause, patient gender, and type of heart failure, monitoring heart failure patients assists in managing symptoms and in aiding clinicians in treatment decisions.
Natriuretic peptides as biomarkers of heart failure
B-type natriuretic peptide (BNP) is a member of a family of endocrine hormones that are well established for use as laboratory tests or biomarkers in diagnosis or exclusion of heart failure and are included in standard of care guidelines for both the American College of Cardiology/American Heart Association (ACC/AHA) and European Society of Cardiology (ESC).16,17
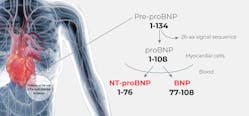
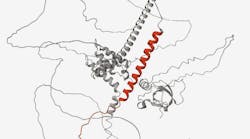
BNP is produced as a prohormone, pre-proBNP, in the myocytes of the cardiac ventricles primarily in response to increased myocardial wallstress and pressure overload that can be brought on by conditions such as heart failure.18–20 Pre-proBNP is cleaved to the precursor peptide, proBNP108, which is subsequently cleaved to form two moieties, the biologically inert 76 amino acid amino-terminal fragment (NT-proBNP) and the biologically active 32 amino acid BNP fragment, in an equimolar ratio.21(See Figure 1.)The biologically active BNP plays an important role in the regulation of the cardiovascular system and control of cardiac function, stimulating the kidneys to excrete sodium, which, in turn, affects blood volume and arterial pressure.22,23 In addition to its role in cardiorenal homeostasis, BNP also reduces vascular resistance and systemic blood pressure, inhibits the renin-angiotensin-aldosterone system, impedes fibrosis, and induces myocardial relaxation.24,25
Elevated natriuretic peptide levels help in identifying those who require further cardiac investigation. Circulating concentrations of both BNP and NT-proBNP have been shown to exhibit clinical utility as measurable biomarkers in patients with suspected heart failure or as prognostic tools for those with existing heart failure.26,27 Patients with higher concentrations of BNP and NT-proBNP have both higher cardiac morbidity and mortality.28 Thus, measuring BNP and NT-proBNP can aid in the diagnosis, severity assessment, and risk stratification of patients with heart failure as well as risk stratification of patients with acute coronary syndrome.11
While both markers are cleared passively by various organs such as the kidneys and liver, BNP is also cleared actively by natriuretic peptide binding receptors and enzyme degradation.23,25,29 As such, BNP has a shorter half-life than NT-proBNP—approximately 20 minutes versus approximately 60–120 minutes, respectively.
Natriuretic peptides and age
Interpreting natriuretic peptide levels must consider the whole patient. Even in healthy individuals, BNP and NT-proBNP levels increase with age and tend to be higher in women than in men.27 Values also increase in patients with atrial fibrillation and renal disease27,30 and decrease in those with obesity.30
In an acute care clinical setting, an NT-proBNP value less than 300 pg/mL and a BNP value less than 100 pg/mL are recommended to rule out acute heart failure with a high degree of certainty.17 (Due to its longer half-life, NT-proBNP values are significantly higher than BNP levels.) Further, use of age-stratified NT-proBNP rule-in thresholds have been shown to improve specificity and positive predictive value (PPV) for diagnosis of heart failure when compared with a single cutoff value.31–33
Comorbidities
Heart failure is a complex medical condition that often coexists with other comorbidities. Further, symptoms of heart failure are nonspecific making heart failure diagnosis difficult. Cautious interpretation of natriuretic peptide concentrations is important in the presence of comorbidities including age, obesity, and renal disease, and conditions such as atrial fibrillation. Results should be interpreted considering the total clinical presentation of the patient, including symptoms, clinical history, data from additional tests, and other appropriate information. For example, obesity may lower NT-proBNP concentrations, while impaired renal function may raise them.16
Heart failure treatments
There are many facets to heart failure treatment. Lifestyle changes include changing diet to increase fiber-rich foods and lowering sugar, salt, caffeine, and alcohol intake. Adding physician-approved exercises can also improve heart failure signs and symptoms. BNP and NT-proBNP are helpful in guiding treatment of heart failure. When treatment is effective, the ventricles shrink, the muscle recovers from stretch, and lower levels of BNP are produced.
Medications such as angiotensin-converting enzyme (ACE) inhibitors, beta blockers, and diuretics may be prescribed to dilate blood vessels, slow heart rate, and reduce fluid, respectively. A newer class of heart failure medicines, Angiotensin Receptor-Neprilysin Inhibitor (ARNi) drugs, is a combination of two drugs—one that blocks angiotensin II and one that breaks down BNP. ARNi drug therapy is designed to dilate blood vessels (angiotensin receptor blocker) and reduce work on the heart (neprilysin inhibitor that aids in sodium removal and blood vessel dilation). Early in ARNi therapy, BNP levels have been shown to increase,34,35 which may make NT-proBNP monitoring more useful in these patients.35
Depending on the severity and etiology of the heart failure, a surgical procedure may be required. Removing arterial blockage and repairing damaged heart valves can improve blood flow within the heart and around the body. Implantable devices, pacemakers and defibrillators, can help with cardiac resynchronization, pacing the heart, and ensuring that dangerous cardiac rhythms are corrected. In situations where the heart damage is severe, patients may require heart transplant. When necessary, ventricular assist devices, mechanical pumping devices, may be used to help pump blood in those awaiting heart transplant.
Closing
It’s been eight years since Louise’s heart attack. With a change in lifestyle, improvement to diet and exercise, and regular monitoring of her natriuretic peptide levels and pacemaker battery level, she is now an active 93-year-old living her life to the fullest.
References
1. Bozkurt B, Coats AJS, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021;23(3):352-380. doi:10.1002/ejhf.2115.
2. Ferreira JP, Kraus S, Mitchell S, et al. World heart federation roadmap for heart failure. Glob Heart. 2019;14(3):197-214. doi:10.1016/j.gheart.2019.07.004.
3. Agnosti F. Heart Failure in the United States. Cardiology Advisor.
4. Lippi G, Sanchis-Gomar F. Global epidemiology and future trends of heart failure. AME Med J. 2020;5:15-15. doi:10.21037/amj.2020.03.03.
5. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13(6):368-378. doi:10.1038/nrcardio.2016.25.
6. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118(17):3272-3287. doi:10.1093/cvr/cvac013.
7. Causes and risk factors. NHLBI, NIH. Accessed June 6, 2023. https://www.nhlbi.nih.gov/health/heart-failure/causes.
8. Hajouli S, Ludhwani D. Heart failure and ejection fraction. In: StatPearls. StatPearls Publishing; 2023.
9. Lam CSP, Solomon SD. The middle child in heart failure: heart failure with mid-range ejection fraction (40-50%). Eur J Heart Fail. 2014;16(10):1049-1055. doi:10.1002/ejhf.159.
10. Andronic AA, Mihaila S, Cinteza M. Heart Failure with Mid-Range Ejection Fraction - a New Category of Heart Failure or Still a Gray Zone. Maedica (Buchar). 2016;11(4):320-324.
11. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129-2200. doi:10.1093/eurheartj/ehw128.
12. Lam CSP, Arnott C, Beale AL, et al. Sex differences in heart failure. Eur Heart J. 2019;40(47):3859-3868c. doi:10.1093/eurheartj/ehz835.
13. Eisenberg E, Di Palo KE, Piña IL. Sex differences in heart failure. Clin Cardiol. 2018;41(2):211-216. doi:10.1002/clc.22917.
14. Lala A, Tayal U, Hamo CE, et al. Sex differences in heart failure. J Card Fail. 2022;28(3):477-498. doi:10.1016/j.cardfail.2021.10.006.
15. Savarese G, Stolfo D, Sinagra G, Lund LH. Heart failure with mid-range or mildly reduced ejection fraction. Nat Rev Cardiol. 2022;19(2):100-116. doi:10.1038/s41569-021-00605-5.
16. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: A report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation. 2022;145(18):e876-e894. doi:10.1161/CIR.0000000000001062.
17. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-3726. doi:10.1093/eurheartj/ehab368.
18. de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362(9380):316-322. doi:10.1016/S0140-6736(03)13976-1.
19. Szabó G. Biology of the B-Type Natriuretic Peptide: Structure, Synthesis and Processing. Biochem Anal Biochem. 2012;01(08). doi:10.4172/2161-1009.1000e129.
20. Nakagawa O, Ogawa Y, Itoh H, et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J Clin Invest. 1995;96(3):1280-1287. doi:10.1172/JCI118162.
21. Kim YS, Karisa N, Jeon WY, Lee H, Kim Y-C, Ahn J. High-level production of N-terminal pro-brain natriuretic peptide, as a calibrant of heart failure diagnosis, in Escherichia coli. Appl Microbiol Biotechnol. 2019;103(12):4779-4788. doi:10.1007/s00253-019-09826-8.
22. 25.8 endocrine regulation of kidney function. Openstax.org. Accessed June 6, 2023. https://openstax.org/books/anatomy-and-physiology-2e/pages/25-8-endocrine-regulation-of-kidney-function.
23. Martinez-Rumayor A, Richards AM, Burnett JC, Januzzi JL. Biology of the natriuretic peptides. Am J Cardiol. 2008;101(3A):3-8. doi:10.1016/j.amjcard.2007.11.012.
24. Vasile VC, Jaffe AS. Natriuretic peptides and analytical barriers. Clin Chem. 2017;63(1):50-58. doi:10.1373/clinchem.2016.254714.
25. Kim H-N, Januzzi JL. Natriuretic peptide testing in heart failure. Circulation. 2011;123(18):2015-2019. doi:10.1161/CIRCULATIONAHA.110.979500.
26. Goetze JP, Bruneau BG, Ramos HR, Ogawa T, de Bold MK, de Bold AJ. Cardiac natriuretic peptides. Nat Rev Cardiol. 2020;17(11):698-717. doi:10.1038/s41569-020-0381-0.
27. Novack ML, Zevitz ME. Natriuretic peptide B type test. In: StatPearls. StatPearls Publishing; 2023.
28. Deswal A. Review: B type natriuretic peptide consistently predicts death and cardiovascular events in heart failure. Evid Based Med. 2005;10(5):150-150. doi:10.1136/ebm.10.5.150.
29. Kavsak PA, Lam CSP, Saenger AK, et al. Educational Recommendations on Selected Analytical and Clinical Aspects of Natriuretic Peptides with a Focus on Heart Failure: A Report from the IFCC Committee on Clinical Applications of Cardiac Bio-Markers. Clin Chem. 2019;65(10):1221-1227. doi:10.1373/clinchem.2019.306621.
30. Chow SL, Maisel AS, Anand I, et al. Role of biomarkers for the prevention, assessment, and management of heart failure: A scientific statement from the american heart association. Circulation. 2017;135(22):e1054-e1091. doi:10.1161/CIR.0000000000000490.
31. Januzzi JL, Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95(8):948-954. doi:10.1016/j.amjcard.2004.12.032.
32. Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27(3):330-337. doi:10.1093/eurheartj/ehi631.
33. Januzzi JL, Chen-Tournoux AA, Christenson RH, et al. N-Terminal Pro-B-Type Natriuretic Peptide in the Emergency Department: The ICON-RELOADED Study. J Am Coll Cardiol. 2018;71(11):1191-1200. doi:10.1016/j.jacc.2018.01.021.
34. Vasquez N, Carter S, Grodin JL. Angiotensin Receptor-Neprilysin Inhibitors and the Natriuretic Peptide Axis. Curr Heart Fail Rep. 2020;17(3):67-76. doi:10.1007/s11897-020-00458-y.
35. Nishikimi T, Nakagawa Y. B-Type Natriuretic Peptide (BNP) Revisited-Is BNP Still a Biomarker for Heart Failure in the Angiotensin Receptor/Neprilysin Inhibitor Era? Biology (Basel). 2022;11(7). doi:10.3390/biology11071034.