The number of infectious disease syndromes commonly seen in primary care, urgent care, and emergency departments in the United States is staggering. Acute respiratory illnesses (ARI), ranging from mild upper respiratory tract infections to serious illnesses such as pneumonia, are the most common reasons to seek ambulatory care1 with total deaths attributed to COVID-19 on death certificates as 1,132,414.2 Gastrointestinal tract (GIT) infections such as acute gastroenteritis have been estimated to account for over 175 million cases each year.3 Sepsis, a serious bloodstream infection, causes up to 381,000 deaths annually.4 Central nervous system (CNS) infections such as meningitis and encephalitis are associated with high mortality and morbidity5 with viral forms responsible for nearly 20,000 U.S. hospitalizations per year.6 The U.S. Centers for Disease Control and Prevention (CDC) reported that 1 in 5 U.S. residents had a sexually transmitted infection (STI) in 2018 which translated to an estimated 26 million new cases that year.7
All these infections may be caused by bacteria, fungi, viruses, parasites, or combinations of two or more of the above and present challenges for accurate diagnosis. Furthermore, many pathogens that require widely different treatment plans produce similar symptoms making global screenings for common suspects more useful than individual or sequential tests when time to treatment is critical. Targeted syndromic, multiplex panels that test numerous pathogens rapidly and simultaneously have the potential to reduce uncertainty in diagnoses, improve antibiotic stewardship, and increase both clinician and patient satisfaction in the process.
There is a growing awareness of the multiple positive impacts and cost savings with the judicious use of multiplex molecular panels for syndromic diagnoses. Molecular panels have been shown to improve sensitivity and detection over conventional culture methods in some cases while reducing the need to collect multiple patient specimens, reducing the turnaround time (TAT) for results, and simplifying the testing algorithm.5 Molecular syndromic panels may be more expensive initially, but they may lower total medical costs by simplifying both clinical and laboratory workflows, improving infection control and prevention, reducing disease progression and morbidity in patients, and reducing unnecessary antibiotic use.
What is syndromic testing?
Syndromic testing refers to multiplex panels that detect common pathogens from patient blood, feces, swabs, and spinal or vaginal fluids using rapid, molecular methods. The molecular panels are either CLIA-waived or considered moderate complexity, which means they can be performed by laboratorians in or near the clinic. The entire panel for a particular kit is assayed and reported to assist in a timely diagnosis, potentially while the patient is still in the clinic. Since many pathogens produce confounding symptoms, molecular panels can eliminate some pathogenic possibilities and help the healthcare provider focus on the true causes of infection.
Respiratory syndromic testing
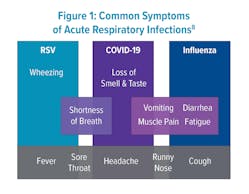
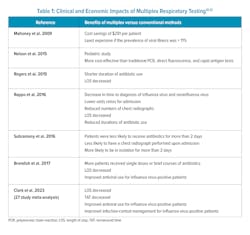
Respiratory diseases such as influenza, respiratory syncytial virus (RSV), and COVID-19 emerge together seasonally and are difficult to separate based on symptoms alone (Figure 1).8 Since the development of polymerase chain reaction (PCR) assays over 40 years ago, respiratory syndromic testing has been evolving to hasten diagnosis using both qualitative and quantitative measures. During the COVID-19 pandemic, the need for quick differentiation for isolation purposes led to the accelerated development of many more molecular multiplex assays.Recent studies have been conducted to assess whether these panels improve patient outcomes, antibiotic stewardship, reduce patient length of stay (LOS), and time to actionable results. Patients with point-of-care (POC) testing for ARI in the emergency department (ED) were more frequently assigned a single room for isolation purposes when having a positive PCR test for either influenza, RSV, or metapneumovirus.9 A 27-study meta-analysis of ED patients found that rapid multiplex PCR for patients with possible ARI reduced LOS and TAT.9 Additionally, the rapid nature of results for influenza-positive patients improved appropriate antiviral usage and infection-control management (Table 1).10
Another study looked at patients with community-acquired pneumonia (CAP). Researchers concluded that molecular panel testing in adults with CAP resulted in a significant reduction in time to actionable results, reduced TAT, and increased microbiological yield compared to standard testing.11 The most frequent pathogens in the CAP study were Haemophilus influenzae, Streptococcus pneumoniae, and influenza A virus. Differentiating between bacterial and viral infections had the potential to improve antibiotic stewardship but this parameter was not quantified in the study.
In a 2018 clinical microbiology review article, the author cited several studies that indicated molecular testing reduced the time to diagnosis of influenza, lowered the odds ratios for hospital admissions, reduced the numbers of chest X-rays, and reduced antibiotic use or shorted the duration of use when compared to conventional methods such as viral cultures, rapid antigen testing, and direct fluorescent-antibody testing (Table 1).12
The cost-effectiveness of respiratory panels is still being debated in the literature. While the reagents and materials required for these assays are more expensive than those for conventional virus testing, multiplex tests may offer savings in the long run if increasing efficiency and reducing labor, time to therapy, the need for admissions, LOS, antibiotic use, and the need for other diagnostic tests is considered.5 More important than dollars and cents; patient’s special needs, disease risks, and final outcomes should be given top priority. Rapid, molecular respiratory panels are particularly useful for high-risk patients such as children, intensive care unit (ICU) patients, transplant recipients, and cystic fibrosis patients.5STI syndromic testing
Many STIs have similar symptoms so empiric treatment based on clinical symptoms alone can cause difficulty in diagnosis and lead to misuse of antibiotics. The increasing rates of STIs require more accurate, rapid POC testing modalities to mitigate the surge.13 But due to lack of highly sensitive rapid testing options, syndromic STI management is prone to high rates of both overtreatment and undertreatment of patients.
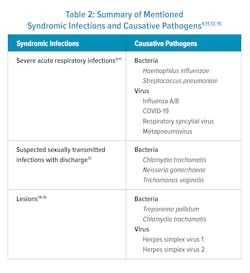
In one recent study comparing a same-day molecular POC test that detected Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis, to off-site molecular tests, it was concluded that implementation of the rapid POC molecular panel could reduce overtreatment and undertreatment of STIs and have minimal impact on staff time and visit duration for patients.13 Patients were willing to wait up to 30 minutes for the results, thus, permitting accurate treatment initiation during a patient visit (Table 2).13
Lesion assays utilizing multiple targets have been able to distinguish between single and multi-pathogen infections with similar presentations. A recent study determined that a molecular multiplex panel was able to definitively diagnose lesions in individuals with suspected syphilis.14 The assay detected a panel of pathogens including the syphilis-causing pathogen Treponema pallidum, as well as other lesion-causing agents — herpes simplex virus (HSV) 1 and 2, and Chlamydia trachomatis serovar L. Their results indicate that over half of syphilis-attributed lesions could be due to other pathogens or causes.14 Another lesion study evaluating three molecular multiplex panels found all three molecular assays were significantly more sensitive for the diagnosis of HSV 1 and 2 lesions compared to viral culture.15 In addition to being more sensitive, molecular methods took 1–2 hours compared to over 2 days for viral culture.15 Utilizing molecular syndromic testing to diagnose clinically similar infections caused by non-descript lesions could improve TAT and reduce misdiagnosis, improper or unnecessary treatment, and the further development of antimicrobial resistance (Table 2).Other syndromic tests
Clinical trials are emerging that point to the utility of GIT, blood, and CNS panels when compared to conventional testing. One multiplex GIT panel was found to reduce LOS from 3.9 to 3.4 days and was estimated to decrease cost of care by approximately $294 per patient.16 Another clinical trial assessing a GIT panel for pathogen detection in cases of infectious diarrhea reported that patients assessed with multiplex PCR were less likely to undergo endoscopy (9.6% versus 8.4%) and were less likely to be prescribed antibiotics (40.9% versus 36.2%).17
When whole blood molecular panels were utilized in the assessment of critically ill patients and compared to Gram stain and MALDI-TOF, the median time to optimal therapy was shortened (14.68 hours to 4.65 hours) and antibiotics were adjusted in 31.8% of patients.18 In 98 hospitalized patients with Gram-negative bacteremia, panels reduced median time to pathogen identification from 30.3 h to 19.1 h with ICU LOS shortened by 4 days and 30-day mortality cut in half.19 The estimated net cost savings per ICU patient was $11,661.19
When evaluating syndromic panels for CNS infections, it was reported that multiplex panels were able to detect significantly more common pathogens than culture, Gram stain, and MALDI-TOF methods.20,21 The same CNS panel was evaluated with 4,623 CSF samples and reported to have 96.3% sensitivity and 96.58% specificity.22
Rapid detection of GIT pathogens with syndromic panels has been demonstrated to improve epidemiological awareness and hasten efforts to prevent further disease transmission to the general public.5 In 2013 and 2014, molecular syndromic testing panels provided the initial recognition of an enterovirus outbreak in one case and led to the identification of additional Shigella cases not found by conventional laboratory assays in another.23,24
Considerations for implementation
Presently, most multiplex panels are limited to the common pathogens capable of causing a specific syndrome.25 Unfortunately, due to differences in patient populations, prevalence of pathogens, and provider ordering patterns, there is no universal or ‘one size fits all’ panel.26 Conversely, some kits may have overstepped their utility by including rare targets that are only found in unique patient populations.26 The menu of analytes on several syndromic panels now offers access to routine testing previously only offered in reference laboratories or for pathogens that are historically difficult to detect.27 By consolidating into panels, laboratories can test from the same samples, often minimizing the need for sequential and more cumbersome, testing methodologies. These workflow changes may improve operational efficiency and cost-effectiveness of testing.27 However, third-party insurers and Medicare contractors have pushed back against certain large panel tests, finding them not medically necessary for most patients; the exceptions being the critically ill and immunosuppressed.26 To remedy this, panels that allow the clinician flexibility in ordering pathogenic targets — a menu approach — may be advised. Clinicians should practice due diligence by continuing to evaluate the utility of panels based on measurable outcomes. Finally, as with all molecular tests, laboratorians and POC testing operators should be properly trained and certified, instruments calibrated and maintained, reagents certified, and methods validated and backed by continuous monitoring to ensure accurate reporting.
Many clinical guidelines do not include syndromic testing at this juncture; guideline publication is often years behind in technological advancements in clinical laboratory medicine. Syndromic testing has been met with broad enthusiasm from patients, clinicians, and laboratory professionals and a few outcome-based studies are showing direct correlation to improved patient care and cost-effectiveness.27 This need for data has driven multiple groups and publications, including the Journal of Clinical Microbiology and the American Society for Microbiology, to call for additional studies to validate anecdotal benefits.27 Publication of patient-outcomes data for syndromic testing, combined with technological advancements in sensitivity and specificity, and the availability of CLIA-waived assays, may lead to inclusion of syndromic testing in future guideline publications.
Summary
To provide utility in the post-pandemic world, infectious disease testing will need to demonstrate speed, accuracy, multiple and simultaneous pathogen detection and quantification, and reach all of the desired measurable outcomes for patients and clinicians alike. The multiplex panels could be tailored to report only specific pathogens of concern which fit the patient’s symptoms. To be flexible and responsive to outbreaks and seasonal fluctuations, multiplex molecular panels could be updated and reengineered by manufacturers to detect emerging or re-emerging pathogens.
Similarly, in the post-pandemic era of laboratory personnel shortages, the ability to run multiplex tests requires fewer laboratory personnel hours, less hands-on time, fewer reagents, and less operational strain on laboratories.28 Laboratorians have cited the positive attributes of molecular syndromic platforms as increased sensitivity, specificity, ease of implementation, and reduced TAT as compared to standard culture or MALDI-TOF assays. Clinicians, in turn, are seeking rapid results that can be obtained during patient visits, targeted treatments based on multiple pathogen screens, improved antibiotic stewardship, and detection of coinfections early in the course of the syndromic disease. At present, many multiplex panels are being evaluated empirically in the clinical setting — especially those that are CLIA-waived or of moderate complexity. It is certain that new and emerging pandemics will spur the development and POC use of these and future syndromic panels.
References
1. National Ambulatory Medical Care Survey: 2013 state and national summary tables. Cdc.gov. Accessed June 20, 2023. https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2013_namcs_web_tables.pdf.
2. NVSS - provisional death counts for COVID-19 - executive summary. Cdc.gov. Published August 8, 2022. Accessed June 20, 2023. https://www.cdc.gov/nchs/covid19/mortality-overview.htm.
3. Wikswo ME, Kambhampati A, Shioda K, et al. Outbreaks of Acute Gastroenteritis Transmitted by Person-to-Person Contact, Environmental Contamination, and Unknown Modes of Transmission--United States, 2009-2013. MMWR Surveill Summ. 2015;11;64(12):1-16. doi:10.15585/mmwr.mm6412a1.
4. Epstein L, Dantes R, Magill S, Fiore A. Varying Estimates of Sepsis Mortality Using Death Certificates and Administrative Codes--United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;8;65(13):342-5. doi:10.15585/mmwr.mm6513a2.
5. Abbott AN, Fang FC. Clinical impact of multiplex syndromic panels in the diagnosis of bloodstream, gastrointestinal, respiratory, and central nervous system infections. Clin Microbiol Newsl. 2017;39(17):133-142. doi:10.1016/j.clinmicnews.2017.08.004.
6. Vora NM, Holman RC, Mehal JM, et al. Burden of encephalitis-associated hospitalizations in the United States, 1998-2010. Neurology. 2014;4;82(5):443-51. doi:10.1212/WNL.0000000000000086.
7. CDC. STI prevalence, incidence, and cost estimates infographic. Centers for Disease Control and Prevention. Published January 25, 2021. Accessed June 20, 2023. https://www.cdc.gov/std/statistics/prevalence-2020-at-a-glance.htm.
8. Diseases & conditions. Mayo Clinic. Accessed June 20, 2023. https://www.mayoclinic.org/diseases-conditions.
9. Bouzid D, Casalino E, Mullaert J, et al. Added value of rapid respiratory syndromic testing at point of care versus central laboratory testing: a controlled clinical trial. J Antimicrob Chemother. 2021;23;76(Supplement_3):iii20-iii27. doi:10.1093/jac/dkab241.
10. Clark TW, Lindsley K, Wigmosta TB, et al. Rapid multiplex PCR for respiratory viruses reduces time to result and improves clinical care: Results of a systematic review and meta-analysis. J Infect. 2023;86(5):462-475. doi:10.1016/j.jinf.2023.03.005.
11. Serigstad S, Markussen D, Grewal HMS, et al. Rapid syndromic PCR testing in patients with respiratory tract infections reduces time to results and improves microbial yield. Sci Rep. 2022;10;12(1):326. doi:10.1038/s41598-021-03741-7.
12. Ramanan P, Bryson AL, Binnicker MJ, Pritt BS, Patel R. Syndromic Panel-Based Testing in Clinical Microbiology. Clin Microbiol Rev. 2017;15;31(1):e00024-17. doi:10.1128/CMR.00024-17.
13. Dawkins M, Bishop L, Walker P, et al. Clinical Integration of a Highly Accurate Polymerase Chain Reaction Point-of-Care Test Can Inform Immediate Treatment Decisions for Chlamydia, Gonorrhea, and Trichomonas. Sex Transm Dis. 2022;1;49(4):262-267. doi:10.1097/OLQ.0000000000001586.
14. Grange PA, Jary A, Isnard C, et al. Use of a Multiplex PCR Assay To Assess the Presence of Treponema pallidum in Mucocutaneous Ulcerations in Patients with Suspected Syphilis. J Clin Microbiol. 2021;21;59(2):e01994-20. doi:10.1128/JCM.01994-20.
15. Slinger R, Amrud K, Sant N, Ramotar K, Desjardins M. A comparison of the Quidel Solana HSV 1 + 2/VZV Assay, the Focus Diagnostics Simplexa HSV 1 & 2 Direct Assay and the Luminex Aries HSV 1&2 Assay for detection of herpes simplex virus 1 and 2 from swab specimens. J Clin Virol. 2019;113:35-38. doi:10.1016/j.jcv.2019.03.002.
16. Beal SG, Tremblay EE, Toffel S, Velez L, Rand KH. A Gastrointestinal PCR Panel Improves Clinical Management and Lowers Health Care Costs. J Clin Microbiol. 2017;26;56(1):e01457-17. doi:10.1128/JCM.01457-17.
17. Axelrad JE, Freedberg DE, Whittier S, et al. Impact of Gastrointestinal Panel Implementation on Health Care Utilization and Outcomes. J Clin Microbiol. 2019;27;57(3):e01775-18. doi:10.1128/JCM.01775-18.
18. Verroken A, Despas N, Rodriguez-Villalobos H, Laterre PF. The impact of a rapid molecular identification test on positive blood cultures from critically ill with bacteremia: A pre-post intervention study. PLoS One. 2019;26;14(9):e0223122. doi:10.1371/journal.pone.0223122.
19. Walker T, Dumadag S, Lee CJ, et al. Clinical Impact of Laboratory Implementation of Verigene BC-GN Microarray-Based Assay for Detection of Gram-Negative Bacteria in Positive Blood Cultures. J Clin Microbiol. 2016;54(7):1789-1796. doi:10.1128/JCM.00376-16.
20. Leber AL, Everhart K, Balada-Llasat JM, et al. Multicenter Evaluation of BioFire FilmArray Meningitis/Encephalitis Panel for Detection of Bacteria, Viruses, and Yeast in Cerebrospinal Fluid Specimens. J Clin Microbiol. 2016;54(9):2251-61. doi:10.1128/JCM.00730-16.
21. Tarai B, Das P. FilmArray® meningitis/encephalitis (ME) panel, a rapid molecular platform for diagnosis of CNS infections in a tertiary care hospital in North India: one-and-half-year review. Neurol Sci. 2019;40(1):81-88. doi:10.1007/s10072-018-3584-y.
22. Pfefferle S, Christner M, Aepfelbacher M, Lütgehetmann M, Rohde H. Implementation of the FilmArray ME panel in laboratory routine using a simple sample selection strategy for diagnosis of meningitis and encephalitis. BMC Infect Dis. 2020;22;20(1):170. doi:10.1186/s12879-020-4904-4.
23. McAllister SC, Schleiss MR, Arbefeville S, et al. Epidemic 2014 enterovirus D68 cross-reacts with human rhinovirus on a respiratory molecular diagnostic platform. PLoS One. 2015 ;23;10(3):e0118529. doi:10.1371/journal.pone.0118529.
24. Prakash VP, LeBlanc L, Alexander-Scott NE, et al. Use of a culture-independent gastrointestinal multiplex PCR panel during a Shigellosis outbreak: considerations for clinical laboratories and public health. J Clin Microbiol. 2015;53(3):1048-9. doi:10.1128/JCM.03374-14.
25. Fox AS, Rao SN. Syndromic testing for the diagnosis of infectious diseases: the right test if used for the right patient. J Antimicrob Chemother. 2021;23;76(Suppl 3):iii2-iii3. doi:10.1093/jac/dkab248.
26. Miller MB. Opinion on Syndromic Panel-Based Testing in Clinical Microbiology. Clin Chem. 2020;1;66(1):42-44. doi:10.1373/clinchem.2019.304832.
27. Clinical utility of multiplex tests for respiratory and GI pathogens. Asm.org. Published August 23, 2019. Accessed June 20, 2023. https://asm.org/Guideline/Clinical-Utility-of-Multiplex-Tests-for-Respirator.
28. Advances and new technologies in syndromic testing. Diagnostics from Technology Networks. Published November 29, 2022. Accessed June 20, 2023. https://www.technologynetworks.com/diagnostics/blog/advances-and-new-technologies-in-syndromic-testing-367947.