Laboratory testing has greatly evolved since its early beginnings in the late 19th century. Education in laboratory medicine for medical trainees has not kept pace with advances in technology, however, leading to uncertainty among physicians on test selection and result interpretation. Laboratory medicine training is not required in the majority of U.S. medical schools, and of those that do, the median time allocated is 12.5 hours.1 Improper test selection and errors in result interpretation are a significant cause of diagnostic errors affecting millions of patients annually.
Diagnostic errors in medicine
One publication suggests that medical errors are the third leading cause of death in the United States but are not listed on death certificates.2 Diagnostic errors are one of four types of errors described in the Institute of Medicine (IOM) report, “To Err is Human: Building a Safer Health System.”3 Some diagnostic errors may result in direct harm while others may lead to delays in establishing a diagnosis. Inappropriate utilization of diagnostic tests, including over- and under-utilization, can contribute to diagnostic errors. In 2015, The IOM published their report, “Improving Diagnosis in Health Care.”4 Designed as a follow up to the 2000 report, this publication highlighted that little had been done to move the needle in terms of diagnostic error detection. This report emphasized the importance of diagnostic error, that diagnostic error had received little attention, and that there was a paucity of good data. Additionally, this report emphasized that making a diagnosis of a disease or condition is a collaborative effort, requiring both intra- and interprofessional teamwork. This report explicitly stated the important role of pathologists, radiologists, and other healthcare personnel who may be forgotten about in the big picture, including medical laboratory scientists.
Data published by the Centers for Disease Control and Prevention reports that an estimated 14 billion laboratory tests are ordered annually in the United States each year by physicians.5 Samples are collected from patients and tested in the more than 260,000 CLIA-certified laboratories that report patient results back to the ordering physician. Given the large volume of tests ordered, undoubtedly there are opportunities for a test to be incorrectly ordered or the results to be interpreted incorrectly. A 2014 survey of 1,768 physicians trained in general internal medicine and family medicine reported uncertainty in test selection and uncertainty in result interpretation 15% and 8% of the time, respectively, in patient diagnostic encounters.6 These self-reports are likely severely underestimated, especially given the significant growth of advanced molecular assays, including next generation sequencing and the use of metagenomics. A frequently described challenge regarding the use of advanced molecular diagnostics such as metagenomics from experts lies in the interpretation of the data generated.7
In fact, a 2021 multicenter study led by Daniel Morgan, MD, evaluated clinicians’ abilities in several scenarios to determine the probability of a variety of disease states before and after laboratory testing.8 Startlingly, they found that clinicians overestimated the probability of disease between 2 to 10 times both before and after testing, and that all clinicians, regardless of training, tended to overestimate disease likelihood. Findings such as these highlight the need for and the importance of diagnostic stewardship approaches in the clinical laboratory.
What is diagnostic stewardship?
Diagnostic stewardship is a term used to refer to initiatives and activities that encompass the entire testing process from pre-analytic to post-analytic phases. It should include input from multiple stakeholders, including experts in pathology and laboratory medicine sub-specialties, who, ideally, lead these efforts. As defined by the World Health Organization, the goal of diagnostic stewardship is to “promote appropriate, timely diagnostic testing, including specimen collection, and pathogen identification and accurate, timely reporting of results to guide patient treatment.”9 In other words, diagnostic stewardship is about using the right test, with the right patient, at the right time. Balancing each of these considerations can be challenging in clinical microbiology, especially given that a large volume of the testing is still culture-based, and final reports will not be available for several days to weeks after the specimen is received in the laboratory.
Use of diagnostic stewardship to improve molecular testing for respiratory viral infections
Like many clinical microbiology laboratories, we moved viral respiratory testing from antigen or large batch runs to syndromic rapid molecular assays with a shortened turnaround time (TAT) in 2014 before the start of the 2014–2015 influenza season. Clinicians were exceedingly happy with the rapid TAT and the breadth of targets included (>20 viral and 3 atypical bacterial pathogens) on the panel. This assay was swapped out for our old one, and there was no education regarding its use to ordering clinicians. Needless to say, use of the test skyrocketed, and after two respiratory seasons we had already spent more than $2 million on test reagents alone.
In May 2017, Palmetto GBA, a Medicare A/B contractor, issued a draft local coverage determination regarding reimbursement for multiplex respiratory panels. The initial draft proposed a non-coverage policy for these assays arguing that they were not medically necessary tests.10 Around the same time, our emergency medicine faculty were seeking a molecular answer for influenza and RSV diagnosis with a more rapid turnaround time in order to maximize patient throughput in our emergency department during the next respiratory season.
The amount of money spent on reagents thus far, the possibility of lack of reimbursement in the future for use of a large molecular assay, and a request for a more rapid test from our largest utilizer of the assay, were signals that a change needed to be made. All of this led us to implement a rapid molecular assay for influenza and RSV. This assay would be the workhorse for the majority of testing, including all testing in our emergency department with results available within 30 minutes from assay start on the analyzer. Testing would be seasonally discontinued when prevalence fell below recommended thresholds for testing. In collaboration with our infectious disease and information services colleagues, we also developed new electronic order sets in our electronic medical record system and developed patient criteria for broad respiratory pathogen panel testing at the start of the 2017–2018 influenza season (Figure 1).The criteria for an expanded respiratory pathogens panel included: 1) Patients with influenza-like illness who were admitted to an inpatient unit, 2) patients who have a medical condition that puts them at higher risk of severe infection (solid organ or bone marrow transplant, AIDS, ongoing receipt of cancer chemotherapy and/or other immunosuppressive medication), and 3) patients who had already received testing for influenza and RSV that was negative. Notably, for adult patients, all three criterion had to be met before expanded respiratory viral testing would be performed. This was guided by the fact that only one-third of all viral respiratory panels in the two previous influenza seasons had been positive for any viral pathogen. Eight percent had been positive for influenza, and four percent for RSV. The remaining positives were for other respiratory pathogens for which directed therapy is not available.
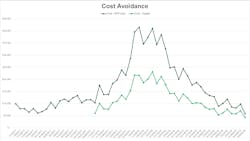
We determined that at our institution, the cost of diagnosing a patient with a viral respiratory illness that was not influenza to be nearly 18 times the cost of diagnosing an episode of bacteremia. At the end of the first year of the diagnostic stewardship program, we calculated the cost avoidance using the implementation of this hybrid approach to testing. We identified that our laboratory saved $321,740 in reagent costs for respiratory virus testing by shifting most of the testing away from our broad respiratory panel (Figure 2). Additional cost savings were also realized in July of 2018 when we adopted a new platform for our expanded respiratory pathogens panel testing.Much of the work on this highlighted examples focused on optimizing test utilization of molecular panels. Specifically, we were focused on using the right test, balancing test volume and cost, testing the right patient, and focusing on appropriate use criteria and test indications at the right time to ensure that a result would be available in time to reasonably impact patient care. Unfortunately, many of the improvements made and attributed to diagnostic stewardship to improve our viral respiratory testing were hindered as a result of the COVID-19 pandemic.
In an ever-evolving healthcare landscape, clinical laboratories need to remain flexible to meet the needs of their patients and the clinicians they serve, all the while being fiscally responsible due to changes related to laboratory regulations and reimbursement. Ensuring that clinical laboratories are thinking about ways to partner with clinical colleagues to develop and implement diagnostic stewardship practices can be used to improve patient care and reduce diagnostic errors.
References
1. Smith BR, Kamoun M, Hickner J. Laboratory Medicine Education at U.S. Medical Schools: A 2014 Status Report. Acad Med. 2016;91(1):107-12. doi:10.1097/ACM.0000000000000817.
2. Makary MA, Daniel M. Medical error-the third leading cause of death in the US. BMJ. 2016;3;353:i2139. doi:10.1136/bmj.i2139.
3. To Err Is Human: Building a Safer Health System. National Academies Press; 2000.
4. Ball JR, Miller BT, Balogh EP, eds. Committee on Diagnostic Error in Health Care, Board on Health Care Services, Institute of Medicine. Improving Diagnosis in Health Care. National Academies Press; 2015.
5. Strengthening clinical laboratories. Cdc.gov. Published November 15, 2018. Accessed April 25, 2023. https://www.cdc.gov/csels/dls/strengthening-clinical-labs.html.
6. J Am Board Fam Med. 2014;27(2):268-74. doi:10.3122/jabfm.2014.02.130104.
7. Johansen Taber KA, Dickinson BD, Wilson M. The promise and challenges of next-generation genome sequencing for clinical care. JAMA Intern Med. 2014;1;174(2):275-80. doi:10.1001/jamainternmed.2013.12048.
8. Morgan DJ, Pineles L, Owczarzak J, Magder L, et al. Accuracy of Practitioner Estimates of Probability of Diagnosis Before and After Testing. JAMA Intern Med. 2021;1;181(6):747-755. doi:10.1001/jamainternmed.2021.0269.
9. Diagnostic stewardship. Who.int. Accessed April 25, 2023. https://apps.who.int/iris/bitstream/handle/10665/251553/WHO-DGO-AMR-2016.3-eng.pdf.
10. Palmetto GBA. MolDX: Multiplex Nucleic Acid Amplified Tests for Respiratory Viral Panels (DL37258). Accessed April 23, 2023. https://www.aphl.org/Materials/Multiplex_Nucleic_Acid_Tests_for_Resp_Viral_Panels_(DL37258)_Palmetto.pdf.