Independent quality control for specialty IA testing
Bio-Rad’s Lyphochek Specialty Immunoassay Control is an independent, human serum based, multi-analyte control that helps support reliable test results in the early detection of chronic diseases. Contains Procalcitonin (PCT), Intact PTH, 25-OH Vitamin D and other complex analytes with increasing clinical utilization for monitoring precision of specialty lab testing.
Ready-to-use reagent kit
Carolina Liquid Chemistries offers the Diazyme EZ Vitamin D Assay, a fast, economical, FDA-cleared liquid ready-to-use reagent kit that recognizes both 25-OH Vitamin D2 and D3 equally. No sample pretreatment required. It offers good correlation with LC-MS/MS and is CLIA moderate complexity on the benchtop Diazyme DZ-Lite c270.
FDA-cleared, automated chemiluminescent assay portfolio
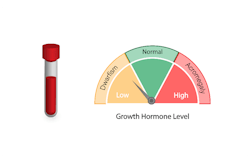
Three FDA-cleared fully automated, chemiluminescent immunoassays which measure human Growth Hormone (hGH), Insulin-like Growth Factor-I (IGF-I), and Insulin-like Growth Factor Binding Protein-3 (IGFBP-3) in human serum or plasma.
The results are used with other clinical and laboratory data to assist the clinician with managing growth hormone deficiency in patients.
Vitamin D for calibration verification
VALIDATE VIT D (Order No. 506RO) is part of our VALIDATE Bone portfolio, offering Vitamin D (VIT D) for easy, fast, efficient calibration verification and linearity, eliminating the need for manual dilutions.
Binding assay
The Elecsys Vitamin D total III binding assay is used for the in vitro quantitative determination of total 25-hydroxyvitamin D to aid in the assessment of vitamin D sufficiency in adults. Results can be produced in as little as 18 minutes on cobas e immunoassay analyzers.
2024 vitamin D proficiency testing program
With WSLH Proficiency Testing, your laboratory can enroll in our Vitamin D proficiency testing program. This program is part of the Immunoassay Chemistry B panel and includes 3 lyophilized serum samples, shipped twice per year. For details, please see PT01710 in our 2024 Clinical PT catalog and price list.