For a printable version of the November CE Story and test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Discuss how vitamin D is metabolized.
2. Describe vitamin D metabolites and their role in overall health and testing for levels of vitamin D.
3. Describe the testing technologies used to measure levels of vitamin D and testing rates worldwide.
4. Describe the potential medical complications of vitamin D deficiency.
Vitamin D is an essential nutrient that is well known for its role in maintaining bone health. Additionally, vitamin D is important in immune function and has been recognized for other health benefits, including reducing the risk of chronic diseases. The most important role of vitamin D is to regulate two key players in bone mineralization — calcium and phosphorus — but it also plays a critical role in many biological functions. Because vitamin D deficiency is associated with numerous increased health risks, the Endocrine Society continues to recommend vitamin D screening for at-risk groups. Understanding the prevalence of vitamin D deficiency and the impact of vitamin D status on overall health can help clinicians provide the appropriate patient care. 1-6
Vitamin D comes in two forms: vitamin D2 (ergocalciferol) is of plant origin, and can be found primarily in supplements, but also in foods such as mushrooms and some greens; vitamin D3 (cholecalciferol) is produced in the skin after exposure to UV light of a specific wavelength (280-315 nm) and can be found in some foods, such as fortified dairy, fish oils, and cod liver oil. The difference between vitamin D2 and vitamin D3 lies in their chemical structure (side chain). Both vitamin D2 and vitamin D3 are biologically inactive prohormones that must be converted to the active vitamin D hormone through a two-step bio-activation process.1,2
Numerous metabolites are created when Vitamin D is activated.
25-Hydroxyvitamin D (25(OH)D)
25(OH)D, or calcidiol, is the form of vitamin D traditionally measured to evaluate vitamin D sufficiency. It is produced when vitamin D2 and D3 pass through the liver and undergo an enzymatic conversion (hydroxylation of carbon 25). The majority of vitamin D (D2 or D3) that is synthesized in the skin or ingested is converted to 25(OH)D; thus, the measurement of serum or plasma 25(OH)D has been adopted as an indicator of an individual’s vitamin D status.7,8
1,25-Dihydroxyvitamin D (1,25(OH)2D)
1,25(OH)2D is the active form of vitamin D. It is obtained upon the enzymatic conversion of 25(OH)D, which occurs primarily in the kidney (hydroxylation of carbon 1). 1,25(OH)2D, also known as calcitriol, maintains mineral homeostasis by enhancing the efficiency of calcium and phosphorus absorption from dietary sources in the small intestine. In the event that dietary calcium supply is not sufficient, 1,25(OH)2D can increase the mobilization of calcium from bone into circulation. Furthermore, 1,25(OH)2D acts on the kidney to preserve calcium by reducing its excretion in urine. 1,25(OH)2D levels are not typically used to evaluate vitamin D status due to the lack of correlation between the metabolite in the blood, and the levels of 25(OH)D or vitamin D. However, 1,25(OH)2D measurement is useful when kidney disease, hyperparathyroidism, sarcoidosis, and other bone and calcium disorders are suspected.7-11
24,25-Dihydroxyvitamin D and 1,24,25-trihydroxyvitamin D
To inactivate and excrete 1,25(OH)2D in the urine, it needs to be transformed from a lipid soluble to a water-soluble molecule. The first step in this conversion is carried out by the 24-hydroxylase enzyme, which produces both 24,25-Dihydroxyvitamin D and 1,24,25-trihydroxyvitamin D. A biological role for 24-hydroxylated vitamin D metabolites remains controversial. While inactivation of the active 1,25(OH)2D makes physiological sense, the role of inactivation of the not-yet-active 25(OH)D has been questioned. However, that 24-hydroxylation of 25(OH)D decreases the available substrate, affording a viable regulatory step.1,2,8,9
C3-epimer of 25(OH)D
The C3-epimer of 25(OH)D is an isomer of 25(OH)D, having the hydroxyl group on carbon 3 in a different spatial plane. It was initially found in infant blood samples, and it can be found at levels similar to or greater than 25(OH)D. Since its initial detection in pediatric samples, it was also detected in adults, albeit less frequently. When present, the C3-epimer is often detected at concentrations below 10 ng/mL, though in extreme cases levels as high as 30-50 ng/mL have been found. To date, no physiological role has been attributed to this metabolite, but increased interest exists to find why some subjects present with such high circulating amounts while others have none.1
Vitamin D assays
Vitamin D status is commonly measured by either commercially available, or lab developed 25(OH)D assays. Methodologies for 25(OH)D measurement include high performance liquid chromatography (HPLC), liquid chromatography-tandem mass spectrometry (LC-MS/MS), radioimmunoassay (RIA), and automated chemiluminescence immunoassay (CLIA).4 HPLC and LC-MS/MS are chemical assay methods that can measure vitamin D metabolites separately. These methods demonstrate excellent analytical specificity and sensitivity. However, the initial capital investment, the labor and time-intensive nature of development, and implementation of clinical LC-MS/MS assays, along with slow turnaround time are key factors hindering greater adoption of this method. Automated immunoassays are the most popular 25(OHD)D tests used by clinical laboratories for high volume testing. Their key advantages over chemical assay methods include relatively low cost, automated sample handling, quick turnaround time, and ease of use.12,13
Discordance in immunoassays has been observed due to differences in 25(OH)D2 and 25(OH)D3 recovery, C3-epimer cross-reactivity, and assay precision. In an effort to standardize 25(OH)D immunoassays, the Center for Disease Control and Prevention established the Vitamin D Standardization Certification Program (VDSCP) to evaluate the accuracy and precision of vitamin D tests and monitor their performance over time. The VDSCP also provides technical support to external quality assurance programs, proficiency testing programs, and research studies. The College of American Pathologists (CAP) and Vitamin D External Quality Assessment Scheme (DEQAS) surveys are two programs used to monitor the performance of laboratories using various methods for 25(OH)D testing.12,14
Potential complications of vitamin D deficiency
As mentioned above, vitamin D deficiency causes imbalance in calcium and phosphorus homeostasis resulting in bone changes. Mineralization defects in the skeleton can cause several bone abnormalities in children (like rickets), and increased bone and muscle pain with consequent risk of falls in adults.5 Low levels of vitamin D are clearly associated with greater illness severity, morbidity and mortality both in children and in adult patients.15
Beyond bones and muscle disorders, low serum 25(OH)D concentrations have been associated with several pathological conditions. In chronic kidney disease, decline of 25(OH)D levels have been associated with worsening of the disease, secondary hyperparathyroidism, low bone mineral density, muscle weakness and risk of falls, metabolic syndrome and obesity, insulin resistance, left ventricular hypertrophy and atherosclerosis, vascular calcification and arterial stiffness, cognitive impairment and mortality.16 In pregnancy, low vitamin D levels have been associated with preeclampsia, gestational diabetes, low birth weight and premature birth.17 Vitamin D insufficiency also has been associated with cardiovascular events like hypertension, coronary artery disease, ischemic heart disease, heart failure, stroke, and type 2 diabetes. However, the causative role of vitamin D was not demonstrated in these conditions.6
Deficiency prevalence worldwide and increased testing rates
Vitamin D insufficiency and deficiency has been observed worldwide. Prevalence of vitamin D deficiency, defined as levels of 25(OH)D lower than 12 ng/mL, was reported in 6% of the population in the U.S., 7.4% in Canada, and 13% in Europe. Vitamin D insufficiency is more common and is reported to be 24% in the U.S., 37% in Canada, and 40% in Europe. Low levels of vitamin D have also been reported in India, Afghanistan, Tunisia and Pakistan with a prevalence higher than 20%.5 Testing frequency has increased significantly in the last 20 years. For example, it increased by 4-fold between 2003 and 2013 in Canada,18 and 42-fold between 2002 and 2017 in Minnesota.19 There is not complete agreement in the world scientific community on the cut-off for insufficiency and deficiency, and a summary of various guidelines worldwide is shown in Table 1. Screening in the general population is not recommended, as current evidence lacks either against or in favor of testing asymptomatic patients.20 However, screening for vitamin D levels is recommended for certain groups of at-risk subjects, which includes people with malnutrition, people with a sedentary lifestyle and obesity, elderly patients, patients with renal, gastrointestinal or liver disease, or people taking medications that alter vitamin D metabolism (anti-seizure, glucocorticoids, AIDS medications, antifungals, cholestyramine).5,21
Impact of vitamin D status on overall health
Even though vitamin D deficiency has been associated with many diseases, the data are conflicting. In many clinical trials, 25(OH)D measurements at baseline or after supplementation are not taken, which confounds outcome analysis on the effects of supplementation on vitamin D deficiency.4 Importantly, vitamin D is a nutrient, and unlike a drug, it is difficult to control in randomized controlled trials because confounders such as habitual intake and sun exposure exist.22 Thus, even though the disease association with vitamin D may exist, cause-effect relationships have been difficult to show. The importance of vitamin D sufficiency may be in early prevention. Studies that analyze available data on the general population — and that focus on productivity, healthcare costs, hospitalization, or mortality, independent of specific disease states — strongly suggest that subjects with adequate levels of vitamin D show less absenteeism,22 have lower health care costs,24-26 and lower mortality. 27, 28
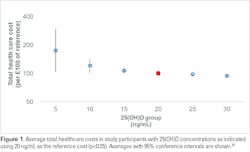
Specifically, a study by Plotnikoff et al. assessed more than 10,000 healthcare employees in the Midwest, and found differences in work performance assessed by the Workplace Productivity and Activity Impairment questionnaire.29 Lower serum 25(OH)D levels were associated with statistically significant lower productivity, with subjects with vitamin D levels >40 ng/mL having the least amount of absenteeism. The authors estimated the potential annual payroll savings at $7.8 million for the employer when its employees achieved 25(OH)D serum levels of 40 ng/mL.23 Furthermore, in healthy populations in northeastern Germany and in veterans in the Southeastern United States, inpatient and outpatient costs were found to be significantly lower in patients with circulating 25(OH)D levels >20 ng/mL (Figure 1).24, 25
The risk of hospitalization was 2-5 times lower in vitamin D sufficient patients as assessed by Kempker et al. in a population of Medicare beneficiaries.30 Hospital length of stay was also shown to be significantly different between mildly and severely deficient patients (5.2 days vs. 13.3 days) in patients from a surgical intensive care unit.28 Cost assessment of hospitalized patients also showed significantly higher costs for the vitamin D-deficient patients.28 Finally, a meta-analysis performed on data from more than 20,000 individuals from the general population in Europe demonstrated a very low risk of mortality in patients with 25(OH)D levels >30 ng/mL. The all-cause mortality risk increased significantly as vitamin D deficiency was more severe.27
Many data on disease association with vitamin D exist, and vitamin D is often believed to be some sort of wonder drug. However, it is perhaps necessary to more strongly emphasize that it is not a drug, but a nutrient, and that its true impact is improved health rather than a cure for disease. There are existing studies that analyze certain variables, such as healthcare costs as surrogate measurement of health. However, more studies are necessary to better understand the true impact of vitamin D on health.
REFERENCES
- Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Bio.2014; 21(3): 319-29. doi: 10.1016/j.chembiol.2013.12.016.
- Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. Vitamin D: Endocrinol Metab Clin North Am. 2010; 39(2): 243-53, table of contents. doi: 10.1016/j.ecl.2010.02.002.
- Christakos S, DeLuca HF. Minireview: Vitamin D: is there a role in extraskeletal health? Endocrinology 2011; 152(8): 2930-6. doi: 10.1210/en.2011-0243.
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009; 19(2): 73-8. doi: 10.1016/j.annepidem.2007.12.001.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96(7): 1911-30. doi: 10.1210/jc.2011-0385.
- Saponaro F, Marcocci C, Zucchi R. Vitamin D status and cardiovascular outcome. J Endocrinol Invest. 2019; 42(11): 1285-90. doi: 10.1007/s40618-019-01057-y.
- DeLuca HF. The vitamin D story: a collaborative effort of basic science and clinical medicine. FASEB J 1988; 2(3): 224-36.
- DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 2004; 80(6 Suppl): 1689S-96S. doi: 10.1093/ajcn/80.6.1689S.
- Deluca HF. History of the discovery of vitamin D and its active metabolites. Bonekey Rep. 2014; 3: 479. doi: 10.1038/bonekey.2013.213.
- Renkema KY, Alexander RT, Bindels RJ, Hoenderop JG. Calcium and phosphate homeostasis: concerted interplay of new regulators. Ann Med 2008; 40(2): 82-91. doi: 10.1080/07853890701689645.
- Valcour A, Zierold C, Podgorski AL, et al. A novel, fully-automated, chemiluminescent assay for the detection of 1,25-dihydroxyvitamin D in biological samples. J Steroid Biochem Mol Biol 2016. 164:120-126. doi: 10.1016/j.jsbmb.2015.08.005.
- Galior K, Ketha H, Grebe S, Singh RJ. 10 years of 25-hydroxyvitamin-D testing by LC-MS/MS-trends in vitamin-D deficiency and sufficiency. Bone rep. 2018; 8: 268-73. doi: 10.1016/j.bonr.2018.05.003.
- Grebe SK, Singh RJ. LC-MS/MS in the clinical laboratory—where to from here? Clin biochem rev 2011; 32(1): 5-31.
- Carter GD, Jones JC, Shannon J, et al. 25-Hydroxyvitamin D assays: Potential interference from other circulating vitamin D metabolites. J Steroid Biochem Mol Biol 2016.164:134-138. doi: 10.1016/j.jsbmb.2015.12.018.
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020; 74(11): 1498-513. doi: 10.1038/s41430-020-0558-y.
- Jean G, Souberbielle JC, Chazot C. Vitamin D in chronic kidney disease and dialysis patients. Nutrients 2017; 9(4): 328. doi: 10.3390/nu9040328.
- De‐Regil LM, Palacios C, Lombardo LK, Peña‐Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane database syst rev. 2016; 14;(1):CD008873. doi: 10.1002/14651858.CD008873.pub3.
- Rodd C, Sokoro A, Lix LM, et al. Increased rates of 25-hydroxy vitamin D testing: Dissecting a modern epidemic. Clin biochem 2018; 59: 56-61. doi: 10.1016/j.clinbiochem.2018.07.005.
- Kerber AA, Pitlick MM, Kellund AE, Weaver AL, Kumar S, Joshi AY. Stable rates of low Vitamin D status among children despite increased testing: a population-based Study. J Pediatr. 2021. 20;S0022-3476(21)00704-6. doi: 10.1016/j.jpeds.2021.07.037.
- LeBlanc ES, Zakher B, Daeges M, Pappas M, Chou R. Screening for vitamin D deficiency: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med.2015; 162(2): 109-22. doi: 10.7326/M14-1659.
- American, Association, Clinical, Endocrinology. Vitamin D deficiency. https://pro.aace.com/disease-state-resources/bone-and-parathyroid/slide-library/vitamin-d-deficiency. Accessed October 1, 2021.
- Boucher BJ. Why do so many trials of vitamin D supplementation fail? Endocr connect 2020; 9(9): R195-r206. doi: 10.1530/EC-20-0274.
- Plotnikoff GA, Finch MD, Dusek JA. Impact of vitamin D deficiency on the productivity of a health care workforce. J Occup Environ Med. 2012; 54(2): 117-21. doi: 10.1097/JOM.0b013e318240df1e.
- Bailey BA, Manning T, Peiris AN. Vitamin D testing patterns among six Veterans Medical Centers in the Southeastern United States: links with medical costs. Mil med. 2012; 177(1): 70-6. doi: 10.7205/milmed-d-11-00204.
- Hannemann A, Wallaschofski H, Nauck M, et al. Vitamin D and health care costs: Results from two independent population-based cohort studies. Clin Nutr. 2018. 37(6 Pt A):2149-2155. doi: 10.1016/j.clnu.2017.10.014.
- Peiris AN, Bailey BA, Manning T. The relationship of vitamin D deficiency to health care costs in veterans. Mil med. 2008; 173(12): 1214-8. doi: 10.7205/milmed.173.12.1214.
- Gaksch M, Jorde R, Grimnes G, et al. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One 2017; 12(2): e0170791. doi: 10.1371/journal.pone.0170791.
- Matthews LR, Ahmed Y, Wilson KL, Griggs DD, Danner OK. Worsening severity of vitamin D deficiency is associated with increased length of stay, surgical intensive care unit cost, and mortality rate in surgical intensive care unit patients. Am J Surg. 2012; 204(1): 37-43. doi: 10.1016/j.amjsurg.2011.07.021.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993; 4(5): 353-65. doi: 10.2165/00019053-199304050-00006.
- Kempker JA, Magee MJ, Cegielski JP, Martin GS. Associations between vitamin D level and hospitalizations with and without an infection in a national cohort of medicare beneficiaries. Am J Epidemiol. 2016; 183(10): 920-9. doi: 10.1093/aje/kwv306.
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Dietary reference intakes for adequacy: calcium and vitamin D. 2011. doi: 10.17226/13050.
- Scientific, Advisory, Committee, on, Nutrition. Vitamin D and Health, 2016.
- Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. Horm Res Paediatr. 2016; 85(2): 83-106. doi: 10.1159/000443136.
- Aspray TJ, Bowring C, Fraser W, et al. National Osteoporosis Society vitamin D guideline summary. Age Ageing 2014; 43(5): 592-5. doi: 10.1093/ageing/afu093.
- Godel JC, Society CP, First Nations I, Committee MH. Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health. 2007; 12(7): 583-98.
- Working, Group, of, et al. Vitamin D and adult bone health in Australia and New Zealand: a position statement. Med J Aust. 2005; 182(6): 281-5. doi: 10.5694/j.1326-5377.2005.tb06701.x.
- Adami S, Romagnoli E, Carnevale V, et al. Guidelines on prevention and treatment of vitamin D deficiency. Reumatismo 2011; 63(3): 129-47. doi: 10.4081/reumatismo.2011.129.