As a woman ages, the consistency of ovulation and/or availability of viable oocytes (eggs) decreases, leading to difficulty in becoming pregnant. The current trend of women choosing to wait to have children until later in life has thus resulted in a larger number of women experiencing infertility and seeking in vitro fertilization (IVF) treatments at fertility clinics. Following an initial consultation with a reproductive endocrinologist, one of the first steps in IVF is an assessment of ovarian reserve — defined as the quantity and quality of remaining oocytes (eggs). The results of this testing can then be used to develop a personalized treatment plan for IVF, including consultation about the likelihood of success. One common tool used to evaluate ovarian reserve is a clinical laboratory test that measures anti-Müllerian hormone (AMH).
What is AMH?
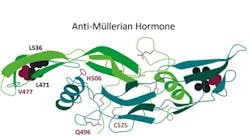
AMH, also known as Müllerian-inhibiting hormone (MIH), is a 140 kDa glycoprotein of the transforming growth factor beta (TGF-β family), produced by the Sertoli cells in males and granulosa cells of developing follicles in females (See Figure 1).
The role of AMH is different in males and females. AMH plays a crucial role in male sex differentiation during fetal development. In males, Sertoli cells secrete AMH during the early stages of development, leading to regression of the Müllerian ducts; hence the name, anti-Mullerian hormone.1 In the absence of AMH, the Müllerian ducts will develop into the uterus and fallopian tubes.
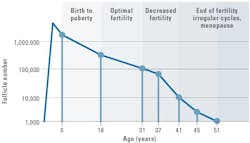
The presence of AMH has been observed as early as 32 weeks gestation in females, and plays a crucial role during folliculogenesis, the maturation of ovarian follicles into mature eggs. Ovarian follicle maturation is on-going; thus, at any given time within the ovary, there are multiple follicles in various stages of development, beginning as primordial follicles and developing into large preovulatory follicles ready for fertilization. A key difference in the formation of male gametes (sperm) and female gametes (eggs) is that women are born with a finite number of eggs averaging approximately 500,000 to 1 million oocytes in the form of primordial follicles.2 Several large studies have demonstrated that the number of primordial follicles immediately declines after birth and approaches nearly zero around age 503 (See Figure 2).4 Throughout the lifetime of a healthy female, only a very small fraction (approximately 0.1%) will develop into a fertilizable egg.
Antral follicle count (AFC) and ovarian reserve
Folliculogenesis begins with the recruitment of a subset of primordial follicles for further development and is regulated by several hormones including follicle-stimulating hormone (FSH), luteinizing hormone (LH), estrogen, and progesterone. The selected primordial follicles grow and develop into primary, secondary, and then antral follicles. During this time, the AMH-producing granulosa cells proliferate to form multiple layers around the oocyte. Ultimately, one follicle becomes dominant and continues to grow and mature in a fertilizable egg while the others generally undergo atresia.
The number of primordial follicles recruited each menstrual cycle is dependent on the overall number in the remaining pool. Although primordial follicles are too small to be visualized, the antral follicles are large enough to be visualized using transvaginal ultrasonography (TVUS)5 and can be counted to estimate ovarian reserve. Antral follicle count (AFC) continues to be the gold standard for assessing ovarian reserve but has limitations, such as the requirement of specialized equipment and highly trained staff. Thus, clinical research has focused on identifying a blood-based biomarker that can be used as a surrogate for AFC.
AMH testing
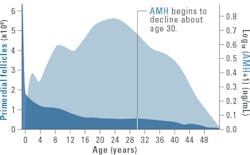
AMH levels have been shown to closely correlate with AFC and have become widely used as a key indicator of ovarian reserve and reproductive potential. Because growing follicles continuously produce AMH (up to ~8mm in size), a decrease in the primordial follicle pool (most often due to naturally occurring fertility changes that happen with aging) will lead to a decrease in the number of growing follicles, and ultimately a decrease in the levels of AMH produced. A higher concentration of AMH means a greater ovarian reserve or number of eggs, while a lower AMH concentration can indicate diminished ovarian reserve or even premature ovarian failure (See Figure 3).6-9
Non-invasive laboratory tests are available to quantitatively measure the amount of AMH in a patient’s blood and provide information on a woman’s ovarian reserve. This becomes important for women presenting to fertility clinics seeking information on their reproductive health. AMH provides advantages over other estimates of ovarian reserve: it’s non-invasive, is less subjective than AFC, which can vary between sonographers, and can be tested at any timepoint throughout the menstrual cycle, unlike FSH. AMH testing also offers wider accessibility, especially in countries or regions with limited resources, as it does not require specialized equipment and highly trained technicians; blood draws can be performed in a variety of healthcare settings. Some in the field predict that AMH may ultimately replace AFC as the gold standard for estimating ovarian reserve.5
Clinical utility in IVF
Systematic reviews and meta-analyses have shown consistent positive association of AMH and AFC with 1) the intensity of ovarian response, 2) oocyte yield, and 3) live birth in IVF cycles.10-13 AMH can be used alongside other hormone testing to predict response to ovarian stimulation during IVF. A very low AMH may indicate premature ovarian failure. This information can be used to inform family planning decisions such as whether to proceed with IVF treatment or pursue other options such as using a donor egg. A low AMH level can also suggest suboptimal response to ovarian stimulation medications administered for IVF whereas as a high AMH may suggest over-response. Using this information, IVF treatment protocols can be tailored to optimize outcomes and reduce the risk of ovarian hyperstimulation syndrome (OHSS), a serious consequence of overstimulation.11,14
PCOS diagnosis & predictability of treatment. A study in 2016 by Anckaert et. al. demonstrated strong correlation between increased levels of AMH and polycystic ovary syndrome (PCOS), nearly 2-3 times higher than that observed in normal individuals.6 AMH elevation in women with PCOS is due to the increased number of small AMH-producing follicles in the ovary. In 2023, the American Society of Reproductive Medicine (ASRM) included AMH as an alternative to ultrasound in the diagnostic algorithm for PCOS in adults.15 AMH can also be used to predict response to laparoscopic ovarian drilling (LOD), a procedure connected to treatment of patients with PCOS. In cases where an individual is diagnosed with PCOS and has high AMH levels (above 8.3 ng/mL), there is a lower chance that they will respond favorably to LOD.16
AMH prediction of menopause
AMH continues to be at the center of ongoing research in reproductive medicine to better understand its role in folliculogenesis, ovarian function, reproductive disorders, and the impact on hormone therapy. AMH levels decline as females age, correlating with the natural decline in ovarian reserve as menopause approaches. Monitoring AMH levels may aid in the prediction of menopause and guide family planning. While several studies have been published, use of AMH in this capacity is not yet clear.17
AMH in research
In other research and clinical settings, AMH has been evaluated for its potential use in evidence-based fertility counseling prior to beginning gender affirming hormone therapy in transgender individuals. This can include counseling on either fertility preservation or contraceptive needs. In transgender men (assigned female at birth and transitioning to male), the administration of testosterone can induce amenorrhea and suppress ovarian function, however unplanned pregnancies have been reported.18 Research focused on transmasculine individuals, have shown AMH remains nearly unchanged during the course of high-testosterone treatment.19 This has given insight to cases where some long-term testosterone users have experienced “surprise” ovulation events. Taub et. al., thereby stress the need for larger studies to confirm or clarify these events.
Final thoughts
AMH is an established, valuable biomarker in reproductive medicine that provides key information used during both fertility assessments and in the evaluation of reproductive disorders. AMH also can help to personalize patient care by tailoring IVF protocols and PCOS treatments to the needs of the individual patient. The utility of this biomarker for other uses continues to be investigated and for example has shown promise for use in the transgender community by identifying the potential for surprise or unplanned pregnancies and may eventually provide answers to the timing of menopause to inform family planning and health management.
References
1. Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14(11):1197-213. doi:10.1038/nm.f.1895.
2. Practice Committee of the American Society for Reproductive Medicine. Electronic address: [email protected]; Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114(6):1151-1157. doi:10.1016/j.fertnstert.2020.09.134.
3. Park CJ, Oh JE, Feng J, et al. Lifetime changes of the oocyte pool: Contributing factors with a focus on ovulatory inflammation. Clin Exp Reprod Med. 2022;49(1):16-25. doi:10.5653/cerm.2021.04917.
4. te Velde ER, Scheffer GJ, Dorland M, Broekmans FJ, Fauser BC. Developmental and endocrine aspects of normal ovarian aging. Mol Cell Endocrinol. 1998;25;145(1-2):67-73. doi:10.1016/s0303-7207(98)00171-3.
5. Fleming R, Seifer DB, Frattarelli JL, Ruman J. Assessing ovarian response: antral follicle count versus anti-Müllerian hormone. Reprod Biomed Online. 2015;31(4):486-96. doi:10.1016/j.rbmo.2015.06.015.
6. Anckaert E, Öktem M, Thies A, et al. Multicenter analytical performance evaluation of a fully automated anti-Müllerian hormone assay and reference interval determination. Clin Biochem. 2016;49(3):260-7. doi:10.1016/j.clinbiochem.2015.10.008.
7. Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7(10):1342-6. doi:10.1093/oxfordjournals.humrep.a137570.
8. Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti-müllerian hormone from conception to menopause. PLoS One. 2011;6(7):e22024. doi:10.1371/journal.pone.0022024.
9. Xu H, Zhang M, Zhang H, et al. Clinical Applications of Serum Anti-Müllerian Hormone Measurements in Both Males and Females: An Update. Innovation (Camb). 2021;9;2(1):100091. doi:10.1016/j.xinn.2021.100091.
10. Broer SL, van Disseldorp J, Broeze KA, et al. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update. 2013;19(1):26-36. doi:10.1093/humupd/dms041.
11. Broer SL, Dólleman M, Opmeer BC, et al. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update. 2011;17(1):46-54. doi:10.1093/humupd/dmq034.
12. Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Müllerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update. 2015;21(6):698-710. doi:10.1093/humupd/dmu062.
13. La Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update. 2014;20(1):124-40. doi:10.1093/humupd/dmt037.
14. Huang J, Lin J, Gao H, et al. Anti-müllerian Hormone for the Prediction of Ovarian Response in Progestin-Primed Ovarian Stimulation Protocol for IVF. Front Endocrinol (Lausanne). 2019;28;10:325. doi:10.3389/fendo.2019.00325.
15. Teede HJ, Tay CT, Laven J, et al. Recommendations from the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Fertil Steril. 2023;120(4):767-793. doi:10.1016/j.fertnstert.2023.07.025.
16. Paramu S. Impact of laparoscopic ovarian drilling on serum anti-mullerian hormone levels in patients with anovulatory Polycystic Ovarian syndrome. Turk J Obstet Gynecol. 2016;13(4):203-207. doi:10.4274/tjod.97523.
17. Nelson SM, Davis SR, Kalantaridou S, et al. Anti-Müllerian hormone for the diagnosis and prediction of menopause: a systematic review. Hum Reprod Update. 2023;2;29(3):327-346. doi:10.1093/humupd/dmac045.
18. Hahn M, Sheran N, Weber S, Cohan D, Obedin-Maliver J. Providing Patient-Centered Perinatal Care for Transgender Men and Gender-Diverse Individuals: A Collaborative Multidisciplinary Team Approach. Obstet Gynecol. 2019;134(5):959-963. doi:10.1097/AOG.0000000000003506.
19. Taub RL, Ellis SA, Neal-Perry G, et al. The effect of testosterone on ovulatory function in transmasculine individuals. Am J Obstet Gynecol. 2020;223(2):229.e1-229.e8. doi:10.1016/j.ajog.2020.01.059.